CARBINOXAMINE MALEATE
02e13b55-2153-468e-a5bd-57dee062f47f
HUMAN PRESCRIPTION DRUG LABEL
Jul 18, 2025
Leading Pharma, LLC
DUNS: 079575060
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
CARBINOXAMINE MALEATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Carbinoxamine maleate is effective for the symptomatic treatment of:
Seasonal and perennial allergic rhinitis. Vasomotor rhinitis.
Allergic conjunctivitis due to inhalant allergens and foods.
Mild, uncomplicated allergic skin manifestations of urticaria and angioedema.
Dermatographism.
As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
Amelioration of the severity of allergic reactions to blood or plasma.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Carbinoxamine maleate is contraindicated in children younger than 2 years of age.
Carbinoxamine maleate is contraindicated in nursing mothers.
Carbinoxamine maleate is contraindicated in patients who are hypersensitive to the drug or on monoamine oxidase inhibitor therapy (seeDrug Interactions).
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
The most frequent adverse reactions are underlined:
Body as a whole: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and throat
Cardiovascular: Hypotension, headache, palpitations, tachycardia, extrasystoles
Hematologic: Hemolytic anemia, thrombocytopenia, agranulocytosis
Central nervous system: Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesia, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions
Gastrointestinal:Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation
Urogenital: Urinary frequency, difficult urination, urinary retention, early menses
Respiratory:Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness
To report SUSPECTED ADVERSE REACTIONS, contact Leading Pharma, LLC at 1-844-740-7500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
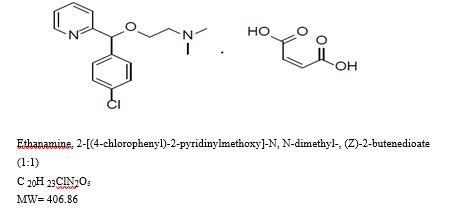
DESCRIPTION
Carbinoxamine maleate is a histamine-H1 receptor blocking agent.
Each immediate release tablet contains 6 mg carbinoxamine maleate for oral administration and the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
Carbinoxamine maleate is very soluble in water, freely soluble in alcohol and in chloroform. Its structure is:
FDA approved dissolution test specifications differ from USP.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Mechanism of Actions
Carbinoxamine maleate, an ethanolamine derivative, is an antihistamine with anticholinergic (drying) and sedative properties. Carbinoxamine appears to compete with histamine (type H1) for receptor sites on effector cells in the gastrointestinal tract, blood vessels and respiratory tract.
Pharmacokinetics and Metabolism
Carbinoxamine is well absorbed from the GI tract and appears to be extensively metabolized by the liver, and excreted in the urine as inactive metabolites within 24 hours. Virtually no intact drug is extended in the urine.
In a study comparing a controlled release suspension and a solution of carbinoxamine, healthy volunteers were administered a single dose of 8 mg carbinoxamine. A time to maximum concentration (Tmax) was between 1.5 hours to 5 hours, a peak plasma concentration (Cmax) of about 24 ng/mL was observed, and extent of exposure (AUC) was about 286 ng hr/mL. The serum half-life is reported to be 10 to 20 hours.
Drug/Food Interactions
Carbinoxamine should not be used in patients with hypersensitivity to carbinoxamine. Carbinoxamine may increase the effects of other drugs such as barbiturates, TCAs, MAO inhibitors such as Phenelzine (Nardil), Tranylcypromine (Parnate), or Selegiline (Eldepryl), alcohol, other antihistamines, and CNS depressants. Carbinoxamine can be taken with or without food.
Cardiovascular Effects
Cardiac effects, including prolongation of QT interval have not been adequately studied. Unlike other newer antihistamines, severe adverse cardiovascular effects are uncommon, and usually limited to over dosage situations.
Special Populations
Pediatric Patients
Carbinoxamine should not be used in newborn or premature infants. Neonates have an increased susceptibility to anticholinergic side effects, such as CNS excitation, which may lead to convulsions.
Pregnancy and Lactation
Safe use of carbinoxamine during pregnancy has not been established. Therefore, carbinoxamine should not be used in women who are, or may become pregnant.
Women who are breast-feeding should avoid use of carbinoxamine, since small amounts appear to be distributed into breast milk.
Geriatric Patients
Carbinoxamine is more likely to cause dizziness, sedation, and hypotension in elderly patients. The incidence of adverse reactions is higher in the elderly; therefore, a dosing adjustment may be necessary in this subpopulation.
WARNINGS SECTION
WARNINGS
Deaths have been reported in children less than 2 years of age who were taking antihistamines, including carbinoxamine-containing drug products, therefore, carbinoxamine maleate is contraindicated in children younger than 2 years of age (seeCONTRAINDICATIONS).
Antihistamines should be used with considerable caution in patients with: narrow angle glaucoma, stenosing peptic ulcer, symptomatic prostatic hypertrophy, bladder neck obstruction, pyloroduodenal obstruction.
PRECAUTIONS SECTION
PRECAUTIONS
General
As many other antihistamines, carbinoxamine maleate has an atropine-like action and, therefore, should be used with caution in patients with: increased intraocular pressure, hyperthyroidism, cardiovascular disease, hypertension.
Antihistamines such as carbinoxamine maleate should not be used to treat lower respiratory tract symptoms, including asthma.
Information for Patients
Carbinoxamine maleate may cause drowsiness; alcohol, sedatives, and tranquilizers may increase the drowsiness effect. Avoid alcoholic beverages while taking this product. Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor. Use caution when driving a motor vehicle or operating machinery.
Drug Interactions
Monoamine oxidase inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines. Carbinoxamine maleate has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.).
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to determine the possible effects of carbinoxamine maleate on carcinogenesis, mutagenesis, and fertility.
Pregnancy
Teratogenic Effects
Animal reproductive studies have not been conducted with carbinoxamine maleate. It is also not known whether carbinoxamine maleate can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Carbinoxamine maleate should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, use of carbinoxamine maleate is contraindicated in nursing mothers (seeCONTRAINDICATIONS).
Pediatric Use
Carbinoxamine maleate is contraindicated in children younger than 2 years of age (seeCONTRAINDICATIONS).
Neonates have an increased susceptibility to anticholinergic side effects, such as CNS excitation, which may lead to convulsions.
Carbinoxamine maleate may diminish mental alertness in children. In the young child, particularly, they may produce excitation.
Geriatric Use
Carbinoxamine maleate is more likely to cause dizziness, sedation, and hypotension in elderly patients (approximately 60 years or older). Sedating drugs may also cause confusion and over sedation in the elderly. Therefore, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
OVERDOSAGE SECTION
OVERDOSAGE
Manifestations
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in children. Atropine-like signs and symptoms- dry mouth; fixed, dilated pupils; flushing; and gastrointestinal symptoms may also occur.
Especially in infants and children, antihistamine overdosage may cause hallucinations, convulsions, or death.
The oral LD50 of carbinoxamine maleate in guinea pigs is 411 mg/kg.
Treatment
The treatment of overdosage with carbinoxamine maleate is essentially symptomatic and supportive. Vital signs (including respiration, pulse, blood pressure, and temperature) and EKG should be monitored. Induction of vomiting is not recommended. Activated charcoal should be given and gastric lavage should be considered after ingestion of a potentially life-threatening amount of drug. In the presence of severe anticholinergic effects, physostigmine may be useful. Vasopressors may be used to treat hypotension.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Carbinoxamine maleate is contraindicated in children younger than 2 years of age (seeCONTRAINDICATIONS). Carbinoxamine maleate should be taken on an empty stomach with water.
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Carbinoxamine maleate dosage should be based on the severity of the condition and the response of the patient. The drug is well tolerated in adults in doses as high as 24 mg daily, in divided doses, over prolonged periods. On the other hand, some patients respond to as little as 4 mg daily.
Clinical experience suggests the following dosage schedule:
Usual Adult Dosage: 1 tablet (6 mg) 3 to 4 times daily
HOW SUPPLIED SECTION
HOW SUPPLIED
Carbinoxamine Maleate Tablets USP, 6 mg are supplied as white to off-white, round tablet, debossed with “LP” over “239” on one side and plain on the other side, in bottles of 100 tablets, NDC 69315-239-01
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container with a child-resistant closure as defined in the official compendium.
Rx only
Manufactured and Distributed by:
Leading Pharma, LLC
3 Oak Rd, Fairfield,
New Jersey (NJ) 07004
United States (USA)
Rev. 04 07/25
