Tetracycline Hydrochloride
Tetracycline Hydrochloride Tablets, USP For Oral Use
c522c860-84ce-4c61-ab72-077de73195df
HUMAN PRESCRIPTION DRUG LABEL
Jan 17, 2024
Pharmaka Generics Inc.
DUNS: 118611093
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Tetracycline Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Tetracycline Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


DESCRIPTION SECTION
DESCRIPTION
Tetracycline is a yellow, crystalline powder. Tetracycline is soluble in water, slightly soluble in ethanol (96%), practically insoluble in acetone. It dissolves in solutions of alkali hydroxides and carbonates. Solutions in water become turbid on standing, owing to the precipitation of tetracycline. The chemical name for tetracycline hydrochloride is 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a octahydro-3,6,10,12,-12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecar- boxamide monohydrochloride. Its structural formula is as follows:
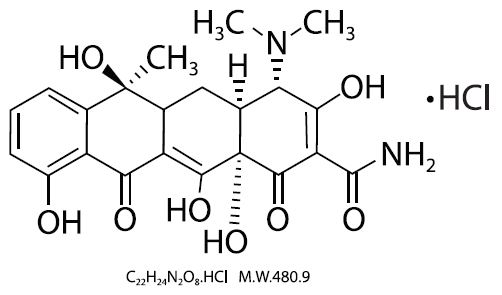
Each tablet, for oral administration, contains 250 mg or 500 mg tetracycline hydrochloride.
Inactive Ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch and stearic acid. The film coating for the 250 mg and 500 mg are made of D&C RED # 30 / helendon pink aluminium lake, hypromellose and titanium dioxide.
In addition to these, the 250 mg tablet film coating includes triacetin and 500 mg tablet film coating includes polyethylene glycol.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Adults: Usual daily dose, 1 gram as 500 mg twice a day or 250 mg four times a day. Higher doses such as 500 mg four times a day may be required for severe infections or for those infections which do not respond to the smaller doses.
For pediatric patients above eight years of age: Usual daily dose, 10 mg/lb to 20 mg/lb (25 mg/kg to 50 mg/kg) body weight divided in four equal doses.
Administration of adequate amounts of fluid with the tablet formulation of tetracycline is recommended to wash down the drug and reduce the risk of esophageal irritation and ulceration (seeADVERSE REACTIONS).
Absorption of tetracycline is impaired by antacids containing aluminum, calcium or magnesium and preparations containing iron, zinc or sodium bicarbonate. Food and some dairy products also interfere with absorption.
When used in streptococcal infections, therapy should be continued for 10 days.
For treatment of brucellosis, 500 mg tetracycline four times a day for three weeks accompanied by streptomycin, 1 gram intramuscularly twice daily the first week and once daily the second week.
For the treatment of syphilis in patients allergic to penicillin, the following dosage of tetracycline is recommended: early syphilis (less than one year's duration), 500 mg four times a day for 15 days. Syphilis of more than one year's duration (except neurosyphilis), 500 mg four times a day for 30 days.
For treatment of gonorrhea, the recommended dose is 500 mg by mouth four times a day for seven days.
Uncomplicated urethral, endocervical or rectal infections in adults caused by Chlamydiatrachomatis: 500 mg, by mouth, four times a day for at least seven days.
In cases of moderate to severe acne which, in the judgement of the clinician, require long-term treatment, the recommended initial dosage is 1 gram daily in divided doses. When improvement is noted, reduce dosage gradually to maintenance levels ranging from 125 mg to 500 mg daily. In some patients it may be possible to maintain adequate remission of lesions with alternate day or intermittent therapy. Tetracycline hydrochloride tablets therapy of acne should augment the other standard measures known to be of value. Duration of long-term treatment which can safely be recommended has not been established (seeWARNINGS andCarcinogenesis, Mutagenesis, Impairment of Fertility).
Use in Specific Population
In patients with renal impairment (seeWARNINGS): decrease total dosage by reduction of recommended individual doses and/or by extending time intervals between doses.
