Major Regular Strength
Major Regular Strength Antacid 150 Chewable Tablets
2ba0eeb2-7048-4259-83d5-9f4ec1c92d32
HUMAN OTC DRUG LABEL
Sep 19, 2025
Major Pharmaceuticals
DUNS: 191427277
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Antacid Tablets
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
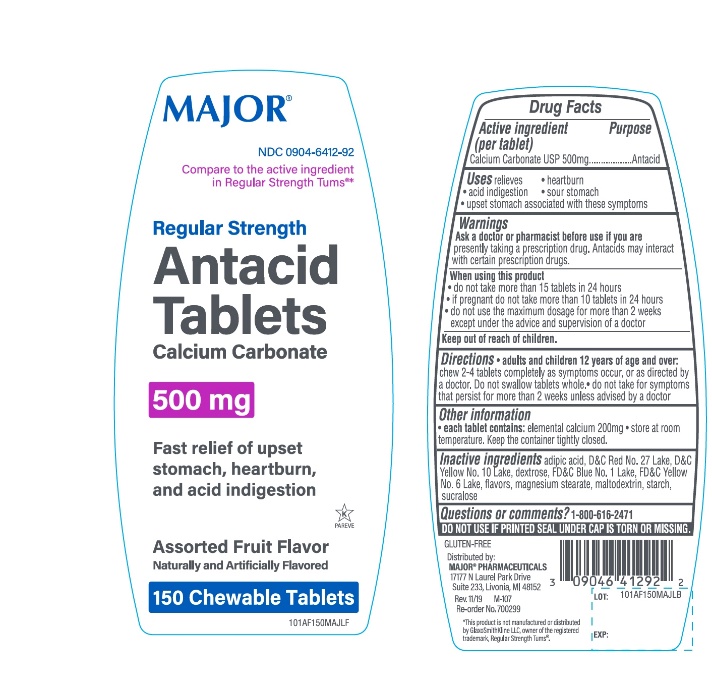
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Principal Display Panel
Compare to active ingredient in Regular Strength Tums ®*
MAJOR®
NDC 0904-6412-92
Regular Strength
Antacid Tablets
Calcium Carbonate 500mg
Fast relief of upset stomach, heartburn, and acid indigestion
Assorted Fruit Flavor
Naturally & Artificially Flavored
150 Chewable Tablets
K PAREVE
GLUTEN- FREE
Distributed by:MAJOR**®**PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48152, USA
M-107 REV. 11/19
*This product is not manufactured or distributed by GlaxoSmithKline LLC, owner of the registered trademark, Regular Strength Tums®.
INDICATIONS & USAGE SECTION
Uses
relieves:
• heartburn
• sour stomach
• acid indigestion
• upset stomach associated with these symptoms
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Calcium Carbonate USP 500mg
OTC - PURPOSE SECTION
Purpose
Antacid
WARNINGS SECTION
Warnings
Ask a doctor or pharmacist before use if you are presentlytaking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- do not take more than 15 tablets in 24 hours
- If pregnant do not take more than 10 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks except under the advice and supervision of a doctor.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of Reach of Children
DOSAGE & ADMINISTRATION SECTION
Directions
***Adults and children 12 years of age and over:**chew 2-4 tablets as symptoms occur, or as directed by a doctor. *Do not swallow tablets whole,
- Do not take for symptoms that persist for more than 2 weeks unless advised by a doctor.
SPL UNCLASSIFIED SECTION
Other Information
- Each tablet contains:elemental calcium 200 mg
- Store at room temperature. Keep the container tightly closed
Safety sealed: Do not use if printed seal under cap is torn or missing.
INACTIVE INGREDIENT SECTION
Inactive ingredients
adipic acid, D&C Red No.27 lake, D&C Yellow No.10 lake, dextrose, FD&C Blue No.1 lake, FD&C Yellow No.6 lake, flavors, magnesium stearate, maltodextrin, starch, sucralose.
