OSELTAMIVIR PHOSPHATE
These highlights do not include all the information needed to use OSELTAMIVIR PHOSPHATE FOR ORAL SUSPENSION safely and effectively. See full prescribing information for OSELTAMIVIR PHOSPHATE FOR ORAL SUSPENSION. OSELTAMIVIR PHOSPHATE for oral suspension Initial U.S. Approval: 1999
7d04f367-8f62-4f63-bac9-717d14f555a5
HUMAN PRESCRIPTION DRUG LABEL
May 2, 2023
Cipla USA, Inc
DUNS: 078719707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
OSELTAMIVIR PHOSPHATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
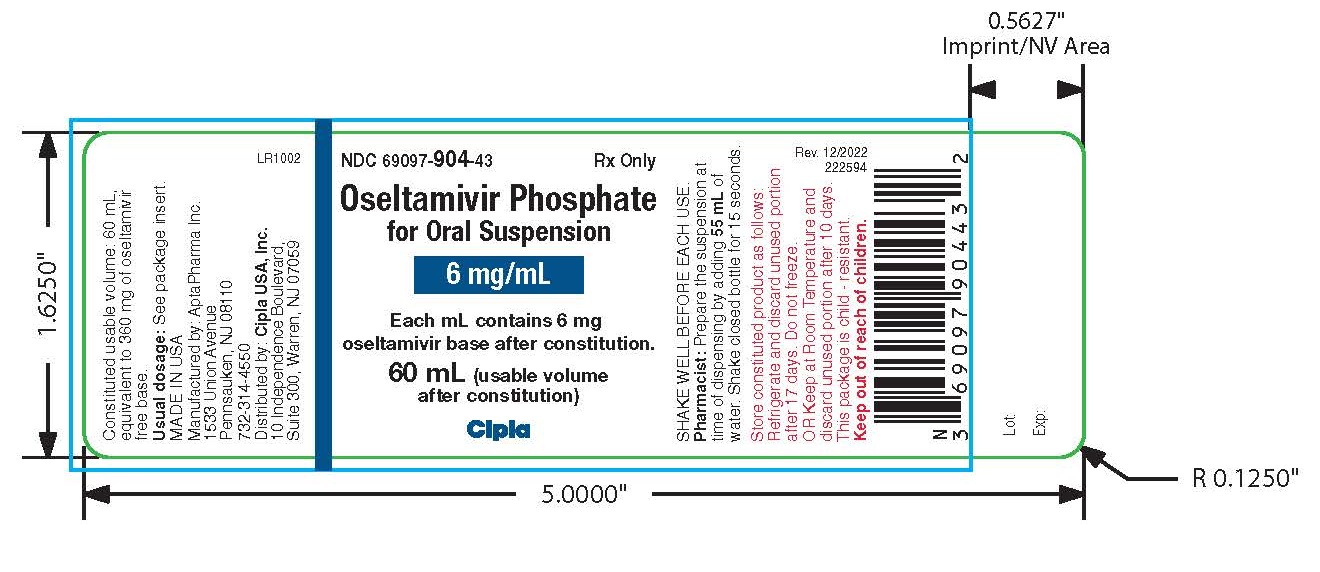
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 69097-904-43
Oseltamivir Phosphate for Oral Suspension
6 mg/mL
Each mL contains 6 mg Oseltamivir base after constitution
60 mL (usable volume after constitution)
Rx Only
Cipla
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Treatment of Influenza
Oseltamivir Phosphate for Oral Suspension is indicated for the treatment of acute, uncomplicated illness due to influenza A and B infection in patients 2 weeks of age and older who have been symptomatic for no more than 48 hours.
1.2 Prophylaxis of Influenza
Oseltamivir Phosphate for Oral Suspension is indicated for the prophylaxis of influenza A and B in patients 1 year and older.
1.3 Limitations of Use
- Oseltamivir Phosphate for Oral Suspension is not a substitute for early influenza vaccination on an annual basis as recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.
- Influenza viruses change over time. Emergence of resistance substitutions could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use Oseltamivir Phosphate for Oral Suspension [see Microbiology (12.4)].
- Oseltamivir Phosphate for Oral Suspension is not recommended for patients with end-stage renal disease not undergoing dialysis [see Dosage and Administration (2.4) and Use in Specific Populations (8.6)].
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Serious Skin/Hypersensitivity Reactions
Cases of anaphylaxis and serious skin reactions including toxic epidermal necrolysis, Stevens-Johnson Syndrome, and erythema multiforme have been reported in postmarketing experience with Oseltamivir Phosphate for Oral Suspension. Stop Oseltamivir Phosphate for Oral Suspension and institute appropriate treatment if an allergic-like reaction occurs or is suspected. The use of Oseltamivir Phosphate for Oral Suspension is contraindicated in patients with known serious hypersensitivity to Oseltamivir Phosphate for Oral Suspension [see Contraindications (4) and Adverse Reactions (6.2)].
5.2 Neuropsychiatric Events
There have been postmarketing reports of delirium and abnormal behavior leading to injury, and in some cases resulting in fatal outcomes, in patients with influenza who were receiving Oseltamivir Phosphate for Oral Suspension [see Adverse Reactions (6.2)]. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made but they appear to be uncommon based on Oseltamivir Phosphate for Oral Suspension usage data. These events were reported primarily among pediatric patients and often had an abrupt onset and rapid resolution. The contribution of Oseltamivir Phosphate for Oral Suspension to these events has not been established. Influenza can be associated with a variety of neurologic and behavioral symptoms that can include events such as hallucinations, delirium, and abnormal behavior, in some cases resulting in fatal outcomes. These events may occur in the setting of encephalitis or encephalopathy but can occur without obvious severe disease. Closely monitor Oseltamivir Phosphate for Oral Suspension-treated patients with influenza for signs of abnormal behavior. If neuropsychiatric symptoms occur, evaluate the risks and benefits of continuing Oseltamivir Phosphate for Oral Suspension for each patient.
5.3 Risk of Bacterial Infections
There is no evidence for efficacy of Oseltamivir Phosphate for Oral Suspension in any illness caused by pathogens other than influenza viruses. Serious bacterial infections may begin with influenza-like symptoms or may coexist with or occur as complications during the course of influenza. Oseltamivir Phosphate for Oral Suspension has not been shown to prevent such complications. Prescribers should be alert to the potential for secondary bacterial infections and treat them as appropriate.
5.4 Fructose Intolerance in Patients with Hereditary Fructose Intolerance
Fructose can be harmful to patients with hereditary fructose intolerance. One dose of 75 mg Oseltamivir Phosphate for Oral Suspension delivers 2 grams of sorbitol. This is above the daily maximum limit of sorbitol for patients with hereditary fructose intolerance, and may cause dyspepsia and diarrhea.
- Serious skin/hypersensitivity reactions such as Stevens-Johnson Syndrome, toxic epidermal necrolysis and erythema multiforme: Discontinue Oseltamivir Phosphate for Oral Suspension and initiate appropriate treatment if allergic-like reactions occur or are suspected. (5.1)
- Neuropsychiatric events: Patients with influenza, including those receiving Oseltamivir Phosphate for Oral Suspension, particularly pediatric patients, may be at an increased risk of confusion or abnormal behavior early in their illness. Monitor for signs of abnormal behavior. (5.2)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in the labeling:
- Serious skin and hypersensitivity reactions [see Warnings and Precautions (5.1)]
- Neuropsychiatric events [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Treatment and Prophylaxis Trials in Adult and Adolescent Subjects (13 years of age and older)
The overall safety profile of Oseltamivir Phosphate for oral Suspension is based on data from 2,646 adult and adolescent subjects that received the recommended dosage of 75 mg orally twice daily for 5 days for treatment of influenza and 1,943 adult and adolescent subjects that received the recommended dosage of 75 mg orally once daily for up to 6 weeks for prophylaxis of influenza in clinical trials.
The most common adverse reactions in the pooled treatment and pooled prophylaxis trials in adults and adolescents are displayed in Table 5. The majority of these adverse reactions were reported on a single occasion, occurred on either the first or second treatment day and resolved spontaneously within 1 to 2 days. This summary includes otherwise healthy adults/adolescents and subjects "at risk" (subjects at higher risk of developing complications associated with influenza, e.g., elderly patients and patients with chronic cardiac or respiratory disease). In general, the safety profile in the subjects "at risk" was qualitatively similar to that in otherwise healthy adults/adolescents.
Table 5 Adverse Reactions Occurring in ≥ 1% of Adults and Adolescents (13 years of age and older) in Treatment and Prophylaxis Trials *
| ||||
|
System Organ Class |
Treatment Trials |
Prophylaxis Trials | ||
|
Oseltamivir phosphate |
Placebo |
Oseltamivir phosphate |
Placebo | |
|
Gastrointestinal Disorders | ||||
|
Nausea |
10% |
6% |
8% |
4% |
|
Vomiting |
8% |
3% |
2% |
1% |
|
Nervous System Disorders | ||||
|
Headache |
2% |
1% |
17% |
16% |
|
General Disorders | ||||
|
Pain |
<1% |
<1% |
4% |
3% |
Adverse Reactions from Treatment and Prophylaxis Trials in Pediatric Subjects (1 year to 12 years of age)
A total of 1,481 pediatric subjects (including otherwise healthy pediatric subjects aged 1 year to 12 years and asthmatic pediatric subjects aged 6 to 12 years) participated in clinical trials of oseltamivir phosphate for the treatment of influenza. A total of 859 pediatric subjects received treatment with Oseltamivir Phosphate for Oral Suspension either at a 2 mg per kg twice daily for 5 days or weight-band dosing. Vomiting was the only adverse reaction reported at a frequency of >1% in subjects receiving Oseltamivir phosphate (16%) compared to placebo (8%).
Amongst the 148 pediatric subjects aged 1 year to 12 years who received oseltamivir phosphate at doses of 30 mg to 60 mg once daily for 10 days in a post-exposure prophylaxis study in household contacts (n = 99), and in a separate 6–week seasonal influenza prophylaxis safety study (n = 49), vomiting was the most frequent adverse reaction (8% on oseltamivir phosphate versus 2% in the no prophylaxis group).
Adverse Reactions from Treatment Trials in Pediatric Subjects (2 weeks to less than 1 year of age)
Assessment of adverse reactions in pediatric subjects 2 weeks to less than 1 year of age was based on two open-label studies that included safety data on 135 influenza-infected subjects 2 weeks to less than 1 year of age (including premature infants at least 36 weeks post conceptional age) exposed to oseltamivir phosphate at doses ranging from 2 mg to 3.5 mg per kg of the formulation for oral suspension twice daily orally for 5 days. The safety profile of oseltamivir phosphate was similar across the age range studied, with vomiting (9%), diarrhea (7%) and diaper rash (7%) being the most frequently reported adverse reactions, and was generally comparable to that observed in older pediatric and adult subjects.
Adverse Reactions from the Prophylaxis Trial in Immunocompromised Subjects
In a 12-week seasonal prophylaxis study in 475 immunocompromised subjects, including 18 pediatric subjects 1 year to 12 years of age, the safety profile in the 238 subjects receiving oseltamivir phosphate 75 mg once daily was consistent with that previously observed in other oseltamivir phosphate prophylaxis clinical trials [see Clinical Studies (14.2)].
Most common adverse reactions (>1% and more common than with placebo):
- Treatment studies – Nausea, vomiting, headache. (6.1)
- Prophylaxis studies – Nausea, vomiting, headache, pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AptaPharma or FDA at 1-732-314-4550 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Influenza Vaccines
Live Attenuated Influenza Vaccine
The concurrent use of oseltamivir phosphate with live attenuated influenza vaccine (LAIV) intranasal has not been evaluated. However, because of the potential for oseltamivir phosphate to inhibit replication of live vaccine virus and possibly reduce the efficacy of LAIV, avoid administration of LAIV within 2 weeks before or 48 hours after oseltamivir phosphate administration, unless medically indicated.
Inactivated Influenza Vaccine
Inactivated influenza vaccine can be administered at any time relative to use of oseltamivir phosphate.
7.2 Drugs Without Clinically Significant Drug Interaction with Oseltamivir
Phosphate for Oral Suspension
No dose adjustments are needed for either oseltamivir or the concomitant drug when coadministering oseltamivir with amoxicillin, acetaminophen, aspirin, cimetidine, antacids (magnesium and aluminum hydroxides and calcium carbonates), rimantadine, amantadine, or warfarin [see Clinical Pharmacology (12.3)]
Live attenuated influenza vaccine (LAIV), intranasal:
Avoid administration of LAIV within 2 weeks before or 48 hours after Oseltamivir Phosphate for Oral Suspension use, unless medically indicated. (7)
DESCRIPTION SECTION
11 DESCRIPTION
Oseltamivir phosphate, USP an influenza neuraminidase inhibitor (NAI), is available as a powder for oral suspension, which when constituted with water as directed contains 6 mg per mL oseltamivir base. In addition to the active ingredient, the powder for oral suspension contains colloidal silicon dioxide, monosodium citrate, saccharin sodium, sodium benzoate, sorbitol, titanium dioxide, tutti-frutti flavoring, and xanthan gum.
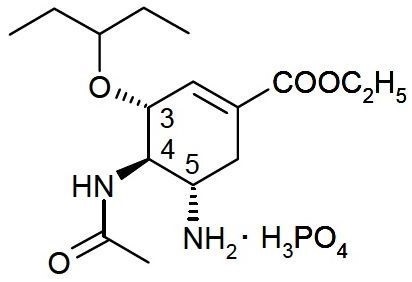
Oseltamivir phosphate, USP is a white to off-white powder with the chemical name (3R,4R,5S)-4-acetylamino-5-amino-3(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid, ethyl ester, phosphate (1:1). The chemical formula is C16H28N2O4 (free base). The molecular weight is 312.4 for oseltamivir free base and 410.4 for oseltamivir phosphate salt. The structural formula is as follows:
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Oseltamivir is an antiviral drug with activity against influenza virus [see Microbiology (12.4)].
12.3 Pharmacokinetics
Absorption and Bioavailability
Oseltamivir is absorbed from the gastrointestinal tract after oral administration of oseltamivir phosphate and is extensively converted predominantly by hepatic esterases to oseltamivir carboxylate. At least 75% of an oral dose reaches the systemic circulation as oseltamivir carboxylate and less than 5% of the oral dose reaches the systemic circulation as oseltamivir (see Table 6).
Table 6 Mean (% CV) Pharmacokinetic Parameters of Oseltamivir and Oseltamivir Carboxylate Following Multiple Dosing of 75 mg Capsules Twice Daily (n=20)|
Parameter |
Oseltamivir |
Oseltamivir Carboxylate |
|
Cmax (ng/mL) |
65 (26) |
348 (18) |
|
AUC0-12h (ng·h/mL) |
112 (25) |
2719 (20) |
Plasma concentrations of oseltamivir carboxylate are proportional to doses up to 500 mg given twice daily (about 6.7 times the maximum recommended Oseltamivir Phosphate for Oral Suspension dosage) [see Dosage and Administration (2)]. Coadministration with food had no significant effect on the peak plasma concentration (551 ng/mL under fasted conditions and 441 ng/mL under fed conditions) and the area under the plasma concentration time curve (6,218 ng∙h/mL under fasted conditions and 6,069 ng∙h/mL under fed conditions) of oseltamivir carboxylate.
Distribution
The volume of distribution (Vss) of oseltamivir carboxylate, following intravenous administration in 24 subjects (Oseltamivir Phosphate for Oral Suspension is not available as an IV formulation), ranged between 23 and 26 liters.
The binding of oseltamivir carboxylate to human plasma protein is low (3%). The binding of oseltamivir to human plasma protein is 42%, which is insufficient to cause significant displacement-based drug interactions.
Elimination
Absorbed oseltamivir is primarily (>90%) eliminated by conversion to the active metabolite, oseltamivir carboxylate. Plasma concentrations of oseltamivir declined with a half-life of 1 to 3 hours in most subjects after oral administration. Oseltamivir carboxylate is not further metabolized and is eliminated unchanged in urine. Plasma concentrations of oseltamivir carboxylate declined with a half-life of 6 to 10 hours in most subjects after oral administration.
Metabolism
Oseltamivir is extensively converted to the active metabolite, oseltamivir carboxylate, by esterases located predominantly in the liver. Oseltamivir carboxylate is not further metabolized. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome P450 isoforms.
Excretion
Oseltamivir carboxylate is eliminated entirely (greater than 99%) by renal excretion. Renal clearance (18.8 L/h) exceeds glomerular filtration rate (7.5 L/h), indicating that tubular secretion (via organic anion transporter) occurs in addition to glomerular filtration. Less than 20% of an oral radiolabeled dose is eliminated in feces.
Specific Populations
Renal Impairment
Administration of 100 mg of oseltamivir phosphate twice daily (about 1.3 times the maximum recommended dosage) for 5 days to subjects with various degrees of renal impairment showed that exposure to oseltamivir carboxylate is inversely proportional to declining renal function.
Population-derived pharmacokinetic parameters were determined for patients with varying degrees of renal function including ESRD patients on hemodialysis. Median simulated exposures of oseltamivir carboxylate for recommended treatment and prophylaxis regimens are provided in Table 7. The pharmacokinetics of oseltamivir have not been studied in ESRD patients not undergoing dialysis [see Indications and Usage (1.3), and Use in Specific Populations (8.6)].
Table 7 Simulated Median Treatment Exposure Metrics of Oseltamivir Carboxylate in Patients with Normal Renal Function, with and Renal Impairment and ESRD Patients on Hemodialysis
| |||||
|
Renal Function/ Impairment |
Normal Creatinine Clearance 90-140 mL/min (n=57) |
Mild Creatinine Clearance 60-90 mL/min (n=45) |
Moderate Creatinine Clearance 30-60 mL/min (n=13) |
Severe Creatinine Clearance 10-30 mL/min (n=11) |
ESRD Creatinine Clearance <10 mL/min on Hemodialysis (n=24) |
|
Recommended Treatment Regimens | |||||
|
PK exposure parameter |
75 mg twice daily |
75 mg twice daily |
30 mg twice daily |
30 mg once daily |
30 mg every HD cycle |
|
Cmin (ng/mL) |
145 |
253 |
180 |
219 |
221 |
|
Cmax (ng/mL) |
298 |
464 |
306 |
477 |
1,170 |
|
AUC48 (ng·h/mL)* |
11224 |
18476 |
12008 |
16818 |
23200 |
|
Recommended Prophylaxis Regimens | |||||
|
PK exposure parameter |
75 mg once daily |
75 mg once daily |
30 mg once daily |
30 mg every other day |
30 mg alternate HD cycle |
|
Cmin (ng/mL) |
39 |
62 |
57 |
70 |
42 |
|
Cmax (ng/mL) |
213 |
311 |
209 |
377 |
903 |
|
AUC48 (ng·hr/mL)* |
5294 |
8336 |
6262 |
9317 |
11200 |
In continuous ambulatory peritoneal dialysis (CAPD) patients, the peak concentration of oseltamivir carboxylate following a single 30 mg dose of oseltamivir or once weekly oseltamivir was approximately 3-fold higher than in patients with normal renal function who received 75 mg twice daily. The plasma concentration of oseltamivir carboxylate on Day 5 (147 ng/mL) following a single 30 mg dose in CAPD patients is similar to the predicted Cmin (160 ng/mL) in patients with normal renal function following 75 mg twice daily. Administration of 30 mg once weekly to CAPD patients resulted in plasma concentrations of oseltamivir carboxylate at the 168 hour blood sample of 63 ng/mL, which were comparable to the Cmin in patients with normal renal function receiving the approved regimen of 75 mg once daily (40 ng/mL).
Hepatic Impairment
In clinical studies, oseltamivir carboxylate exposure was not altered in subjects with mild or moderate hepatic impairment [see Use in Specific Populations (8.7)].
Pregnant Women
A pooled population pharmacokinetic analysis indicates that the Oseltamivir Phosphate for Oral Suspension dosage regimen resulted in lower exposure to the active metabolite in pregnant women (n=59) compared to non-pregnant women (n=33). However, this predicted exposure is expected to have activity against susceptible influenza virus strains and there are insufficient pharmacokinetics and safety data to recommend a dose adjustment for pregnant women [see Use in Specific Populations (8.1)].
Pediatric Subjects (1 year to 12 years of age)
The pharmacokinetics of oseltamivir and oseltamivir carboxylate have been evaluated in a single-dose pharmacokinetic study in pediatric subjects aged 5 to 16 years (n=18) and in a small number of pediatric subjects aged 3 to 12 years (n=5) enrolled in a clinical trial. Younger pediatric subjects cleared both the prodrug and the active metabolite faster than adult subjects resulting in a lower exposure for a given mg/kg dose. For oseltamivir carboxylate, apparent total clearance decreases linearly with increasing age (up to 12 years). The pharmacokinetics of oseltamivir in pediatric subjects over 12 years of age are similar to those in adult subjects [see Use in Specific Populations (8.4)].
Pediatric Subjects (2 weeks to less than 1 year of age)
The pharmacokinetics of oseltamivir and oseltamivir carboxylate have been evaluated in two open-label studies of pediatric subjects less than one year of age (n=122) infected with influenza. Apparent clearance of the active metabolite decreases with decreasing age in subjects less than 1 year of age; however the oseltamivir and oseltamivir carboxylate exposure following a 3 mg/kg dose in subjects under 1 year of age is expected to be within the observed exposures in adults and adolescents receiving 75 mg twice daily and 150 mg twice daily [see Use in Specific Populations (8.4)].
Geriatric Patients
Exposure to oseltamivir carboxylate at steady-state was 25% to 35% higher in geriatric subjects (age range 65 to 78 years) compared to young adults given comparable doses of oseltamivir. Half-lives observed in the geriatric subjects were similar to those seen in young adults. Based on drug exposure and tolerability, dose adjustments are not required for geriatric patients for either treatment or prophylaxis [see Use in Specific Populations (8.5)].
Drug Interaction Studies
Oseltamivir is extensively converted to oseltamivir carboxylate by esterases, located predominantly in the liver. Drug interactions involving competition for esterases have not been extensively reported in literature. Low protein binding of oseltamivir and oseltamivir carboxylate suggests that the probability of drug displacement interactions is low.
In vitro studies demonstrate that neither oseltamivir nor oseltamivir carboxylate is a good substrate for P450 mixed-function oxidases or for glucuronyl transferases.
Coadministration of probenecid results in an approximate two-fold increase in exposure to oseltamivir carboxylate due to a decrease in active anionic tubular secretion in the kidney. However, due to the safety margin of oseltamivir carboxylate, no dose adjustments are required when coadministering with probenecid. No clinically relevant pharmacokinetic interactions have been observed when coadministering oseltamivir with amoxicillin, acetaminophen, aspirin, cimetidine, antacids (magnesium and aluminum hydroxides and calcium carbonates), rimantadine, amantadine, or warfarin.
12.4 Microbiology
Mechanism of Action
Oseltamivir phosphate is an ethyl ester prodrug requiring ester hydrolysis for conversion to the active form, oseltamivir carboxylate. Oseltamivir carboxylate is an inhibitor of influenza virus neuraminidase affecting release of viral particles. The median IC50 values of oseltamivir against influenza A/H1N1, influenza A/H3N2, and influenza B clinical isolates were 2.5 nM (range 0.93-4.16 nM, N=74), 0.96 nM (range 0.13-7.95 nM, N=774), and 60 nM (20-285 nM, N=256), respectively, in a neuraminidase assay with a fluorescently labeled MUNANA substrate.
Antiviral Activity
The antiviral activity of oseltamivir carboxylate against laboratory strains and clinical isolates of influenza virus was determined in cell culture. The concentrations of oseltamivir carboxylate required for inhibition of influenza virus in cell culture were highly variable depending on the assay method used and the virus tested. The 50% and 90% effective concentrations (EC50 and EC90) were in the range of 0.0008 micromolar to greater than 35 micromolar and 0.004 micromolar to greater than 100 micromolar, respectively (1 micromolar=0.284 microgram per mL). The relationship between the antiviral activity in cell culture, inhibitory activity in the neuraminidase assay, and the inhibition of influenza virus replication in humans has not been established.
Resistance
Cell culture studies: Influenza A virus isolates with reduced susceptibility to oseltamivir carboxylate have been recovered by serial passage of virus in cell culture in the presence of increasing concentrations of oseltamivir carboxylate. Reduced susceptibility of influenza virus to inhibition by oseltamivir carboxylate may be conferred by amino acid substitutions in the viral neuraminidase and/or hemagglutinin proteins.
Clinical studies: Reduced susceptibility isolates have been obtained during treatment with oseltamivir and from sampling during community surveillance studies. Changes in the viral neuraminidase that have been associated with reduced susceptibility to oseltamivir carboxylate are summarized in Table 8. The clinical impact of this reduced susceptibility is unknown.
Hemagglutinin (HA) substitutions selected in cell culture and associated with reduced susceptibility to oseltamivir include (influenza virus subtype- specific numbering) A11T, K173E, and R453M in H3N2; and H99Q in influenza B virus (Yamagata lineage). In some cases, HA substitutions were selected in conjunction with known NA resistance substitutions and may contribute to reduced susceptibility to oseltamivir; however, the impact of HA substitutions on antiviral activity of oseltamivir in humans is unknown and likely to be strain-dependent.
Table 8 Neuraminidase Amino Acid Substitutions Associated with Reduced Susceptibility to Oseltamivir|
Amino Acid Substitution* |
|---|
|
|
Influenza A N1 (N1 numbering in brackets) |
|
I117V (I117V), E119V (E119V), R152K (R152K), Y155H (Y155H), F173V (F174V), D198G/N (D199G/N), I222K/R/T/V (I223K/R/T/V), S246N (S247N), G248R+I266V (G249R+I267V), H274Y (H275Y), N294S (N295S), Q312R+I427T (Q313R+I427T), N325K (N325K), R371K (R368K) |
|
Influenza A N2 |
|
E41G, E119I/V, D151V, I222L/V, Q226H, SASG245-248 deletion, S247P, R292K, N294S |
|
Influenza B (B numbering in brackets) |
|
E119A (E117A), P141S (P139S), G142R (G140R), R152K (R150K), D198E/N/Y (D197E/N/Y), I222L/T/V (I221L/T/V), A246D/S/T (A245D/S/T), H274Y (H273Y), N294S (N294S), R371K (R374K), G402S (G407S) |
Selection of influenza A viruses resistant to oseltamivir can occur at higher frequencies in children. The incidence of Oseltamivir treatment-associated resistance in pediatric treatment studies has been detected at rate of 27% to 37% and 3% to 18% (3/11 to 7/19 and 1/34 to 9/50 post-treatment isolates, respectively) for influenza A/H1N1 virus and influenza A/H3N2 virus, respectively.
The frequency of resistance selection to oseltamivir and the prevalence of such resistant virus vary seasonally and geographically.
Circulating seasonal influenza strains expressing neuraminidase resistance- associated substitutions have been observed in individuals who have not received oseltamivir treatment. The oseltamivir resistance-associated substitution H275Y was found in more than 99% of US-circulating 2008 H1N1 influenza virus isolates. The 2009 H1N1 influenza virus ("swine flu") was almost uniformly susceptible to oseltamivir; however, the frequency of circulating resistant variants can change from season to season. Prescribers should consider available information from the CDC on influenza virus drug susceptibility patterns and treatment effects when deciding whether to use Oseltamivir Phosphate for Oral Suspension.
Cross-resistance
Cross-resistance between oseltamivir and zanamivir has been observed in neuraminidase biochemical assays. The H275Y (N1 numbering) or N294S (N2 numbering) oseltamivir resistance-associated substitutions observed in the N1 neuraminidase subtype, and the E119V or N294S oseltamivir resistance- associated substitutions observed in the N2 subtype (N2 numbering), are associated with reduced susceptibility to oseltamivir but not zanamivir. The Q136K and K150T zanamivir resistance-associated substitutions observed in N1 neuraminidase, or the S250G zanamivir resistance-associated substitutions observed in influenza B virus neuraminidase, confer reduced susceptibility to zanamivir but not oseltamivir. The R292K oseltamivir resistance-associated substitution observed in N2, and the I222T, D198E/N, R371K, or G402S oseltamivir resistance-associated substitutions observed in influenza B virus neuraminidase, confer reduced susceptibility to both oseltamivir and zanamivir. These examples do not represent an exhaustive list of cross resistance-associated substitutions and prescribers should consider available information from the CDC on influenza drug susceptibility patterns and treatment effects when deciding whether to use Oseltamivir Phosphate for Oral Suspension.
No single amino acid substitution has been identified that could confer cross- resistance between the neuraminidase inhibitor class (oseltamivir, zanamivir) and the M2 ion channel inhibitor class (amantadine, rimantadine). However, a virus may carry a neuraminidase inhibitor associated substitution in neuraminidase and an M2 ion channel inhibitor-associated substitution in M2 and may therefore be resistant to both classes of inhibitors. The clinical relevance of phenotypic cross-resistance evaluations has not been established.
Immune Response
No influenza vaccine/oseltamivir interaction study has been conducted. In studies of naturally acquired and experimental influenza, treatment with Oseltamivir Phosphate for Oral Suspension did not impair normal humoral antibody response to infection.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year carcinogenicity studies in mice and rats given daily oral doses of the prodrug oseltamivir phosphate up to 400 mg/kg and 500 mg/kg, respectively, the prodrug and the active form oseltamivir carboxylate induced no statistically significant increases in tumors over controls. The mean maximum daily exposures to the prodrug in mice and rats were approximately 130- and 320-fold, respectively, greater than those in humans at the recommended clinical dose based on AUC comparisons. The respective safety margins of the exposures to the active oseltamivir carboxylate were 15- and 50-fold.
Oseltamivir was found to be non-mutagenic in the Ames test and the human lymphocyte chromosome assay with and without enzymatic activation and negative in the mouse micronucleus test. It was found to be positive in a Syrian Hamster Embryo (SHE) cell transformation test. Oseltamivir carboxylate was non-mutagenic in the Ames test and the L5178Y mouse lymphoma assay with and without enzymatic activation and negative in the SHE cell transformation test.
In a fertility and early embryonic development study in rats, doses of oseltamivir at 50, 250, and 1500 mg/kg/day were administered to females for 2 weeks before mating, during mating and until day 6 of pregnancy. Males were dosed for 4 weeks before mating, during mating, and for 2 weeks after mating. There were no effects on fertility, mating performance or early embryonic development at any dose level. The highest dose in this study was approximately 115 times the human systemic exposure (AUC0-24h) of oseltamivir carboxylate that occurs after administration of the maximum recommended human dose.
PATIENT COUNSELING INFORMATION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Skin/Hypersensitivity Reactions
Advise patients and/or caregivers of the risk of severe allergic reactions (including anaphylaxis) or serious skin reactions. Instruct patients and/or caregiver to stop Oseltamivir Phosphate for Oral Suspension and seek immediate medical attention if an allergic-like reaction occurs or is suspected [see Warnings and Precautions (5.1)].
Neuropsychiatric Events
Advise patients and/or caregivers of the risk of neuropsychiatric events in Oseltamivir Phosphate for Oral Suspension-treated patients with influenza and instruct patients to contact their physician if they experience signs of abnormal behavior while receiving Oseltamivir Phosphate for Oral Suspension [see Warnings and Precautions (5.2)].
Important Dosing Information
Instruct patients to begin treatment with Oseltamivir Phosphate for Oral Suspension as soon as possible from the first appearance of flu symptoms, within 48 hours of onset of symptoms. Similarly, instruct patients to start taking Oseltamivir Phosphate for Oral Suspension for prevention as soon as possible after exposure [see Dosage and Administration (2)]. Instruct patients to take any missed doses as soon as they remember, except if it is near the next scheduled dose (within 2 hours), and then continue to take Oseltamivir Phosphate for Oral Suspension at the usual times.
Influenza Vaccines
Instruct patients that Oseltamivir Phosphate for Oral Suspension is not a substitute for receiving an annual flu vaccination. Patients should continue receiving an annual flu vaccination according to guidelines on immunization practices. Because of the potential for Oseltamivir Phosphate for Oral Suspension to inhibit replication of live attenuated influenza vaccine (LAIV) and possibly reduce efficacy of LAIV, avoid administration of LAIV within 2 weeks or 48 hours after Oseltamivir Phosphate for Oral Suspension administration, unless medically necessary [see Drug Interactions (7.1)].
Fructose Intolerance
Inform patients with hereditary fructose intolerance that one dose of 75 mg Oseltamivir Phosphate for Oral Suspension (supplied as powder) delivers 2 grams of sorbitol. Inform patients with hereditary fructose intolerance that this is above the daily maximum limit of sorbitol and may cause dyspepsia and diarrhea [see Warnings and Precautions (5.4)].
Manufactured by:
AptaPharma Inc.,
Pennsauken, NJ 08110 USA
PI-1002
Distributed by:
Cipla USA, Inc,
10 Independence Boulevard, Suite 300
Warren, NJ 07059
Rev. 12/2022
PATIENT MEDICATION INFORMATION SECTION
PATIENT INFORMATION
Oseltamivir Phosphate for oral suspension
(oh'' sel tam' i vir fos' fate)
|
What is Oseltamivir Phosphate for Oral Suspension? Oseltamivir Phosphate for Oral Suspension is a prescription medicine used to:
It is not known if Oseltamivir Phosphate for Oral Suspension is:
Oseltamivir Phosphate for Oral Suspension does not treat or prevent illness that is caused by infections other than the influenza virus. Oseltamivir Phosphate for Oral Suspension does not prevent bacterial infections that may happen with the flu. Oseltamivir Phosphate for Oral Suspension is not recommended for people with end-stage renal disease (ESRD) who are not receiving dialysis. **Oseltamivir Phosphate for Oral Suspension does not take the place of receiving a flu vaccination. Talk to your healthcare provider about when **you should receive an annual flu vaccination. Who should not take Oseltamivir Phosphate for Oral Suspension? Do not take Oseltamivir Phosphate for Oral Suspension if you are allergic to oseltamivir phosphate or any of the ingredients in Oseltamivir Phosphate for Oral Suspension.See the end of this leaflet for a complete list of ingredients in Oseltamivir Phosphate for Oral Suspension. What should I tell my healthcare provider before taking Oseltamivir Phosphate for Oral Suspension? Before you take Oseltamivir Phosphate for Oral Suspension, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription or over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. How should I take Oseltamivir Phosphate for Oral Suspension?
If your healthcare provider or pharmacist has instructed you to take Oseltamivir Phosphate for Oral Suspension, read the detailed Instructions for Use at the end of this leaflet. Ask your pharmacist if you have any questions. What are the possible side effects of Oseltamivir Phosphate for Oral Suspension? Oseltamivir Phosphate for Oral Suspension may cause serious side effects, including: *Serious skin and allergic reactions. Oseltamivir Phosphate for Oral Suspension can cause serious skin and allergic reactions. Stop taking Oseltamivir Phosphate for Oral Suspension and get medical help right away if you get any of the following symptoms:
The most common side effects of Oseltamivir Phosphate for Oral Suspension when used for treatment of the flu include nausea, vomiting, and headache. The most common side effect of Oseltamivir Phosphate for Oral Suspension when used for prevention of the flu include nausea, vomiting, headache, and pain. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of Oseltamivir Phosphate for Oral Suspension. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store Oseltamivir Phosphate for Oral Suspension?
Oseltamivir Phosphate for Oral Suspension comes in a child-resistant package. Keep Oseltamivir Phosphate for Oral Suspension and all medicines out of the reach of children. General information about the safe and effective use of Oseltamivir Phosphate for Oral Suspension. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Oseltamivir Phosphate for Oral Suspension for a condition for which it was not prescribed. Do not give Oseltamivir Phosphate for Oral Suspension to other people, even if they have the same symptoms you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Oseltamivir Phosphate for Oral Suspension that is written for health professionals. For more information contact AptaPharma at 1-732-314-4550. What are the ingredients in Oseltamivir Phosphate for Oral Suspension? Active ingredient: Oseltamivir Phosphate, USP Inactive ingredients: Oseltamivir Phosphate for Oral Suspension: Colloidal silicon dioxide, monosodium citrate, saccharin sodium, sodium benzoate, sorbitol, titanium dioxide, tutti-frutti flavoring, and xanthan gum. Manufactured by: AptaPharma Inc., Pennsauken, NJ 08110 USA PI-1002 Distributed by: Cipla USA, Inc, 10 Independence Boulevard, Suite 300 Warren, NJ 07059 |
|
This Instructions for Use has been approved by the U.S. Food and Drug Administration. |
Revised: 12/2022
INSTRUCTIONS FOR USE SECTION
INSTRUCTIONS FOR USE
Oseltamivir Phosphate for oral suspension
(oh'' sel tam' i vir fos' fate)
How do I give a dose of Oseltamivir Phosphate for Oral Suspension?
|
Step 1. |
Shake the Oseltamivir Phosphate for oral suspension bottle well before each use. |
|
Step 2. |
Open the bottle by pushing downward on the child resistant bottle cap and twisting it in the direction of the arrow. |
|
Step 3. |
Measure the oral suspension with an appropriate oral dosing dispenser to be sure you get the correct dose. Contact your pharmacist if you do not have an appropriate oral dosing dispenser. |
|
Step 4. |
Give the full contents of oral dosing dispenser directly into the mouth. |
|
Step 5. |
Close the bottle with the child-resistant bottle cap after each use. |
|
Step 6. |
Rinse oral dosing dispenser under running tap water and allow to air dry after each use. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 12/2022
