SODIUM CITRATE AND CITRIC ACID
SODIUM CITRATE AND CITRIC ACID ORAL SOLUTION, USP500 mg-334 mg/5 mL (1,500 mg-1,002 mg/15 mL Unit Dose)500 mg-334 mg/5 mL (3,000 mg-2,004 mg/30 mL Unit Dose)Dietary Supplement - FOR INSTITUTIONAL USE ONLY• Alcohol Free• Sugar Free• Cherry Flavor
37ebacdd-1964-45c3-b2e2-2f835ca64af8
DIETARY SUPPLEMENT
May 15, 2025
Rising Pharma Holdings, Inc.
DUNS: 116880195
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SODIUM CITRATE AND CITRIC ACID
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
500 mg-334 mg/5 mL (1,500 mg-1,002 mg/15 mL Unit Dose)
Case label -
57237-319-52 (100 x 15 mL Unit-Dose Cups)

Lid Label -
57237-319-15 (Delivers 15 mL)

500 mg-334 mg/5 mL (3,000 mg-2,004 mg/30 mL Unit Dose)
Case label -
57237-319-31 (100 x 30 mL Unit-Dose Cups)
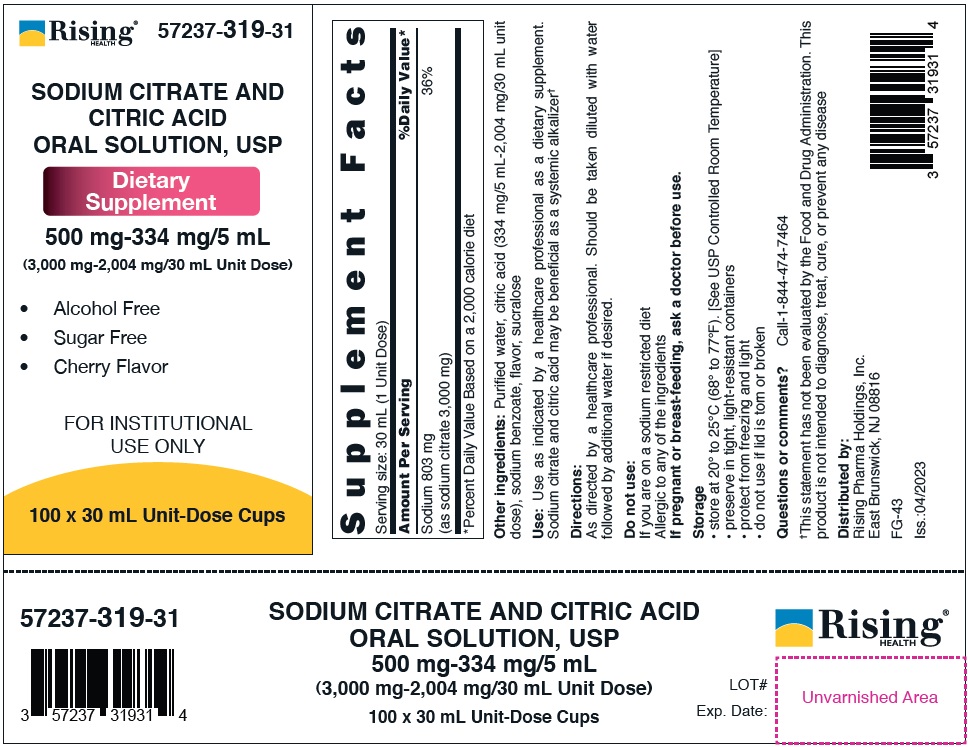
Lid Label -
57237-319-03 (Delivers 30 mL)

STATEMENT OF IDENTITY SECTION
|
Serving Size: 15 mL (1 Unit Dose) | |
|
Amount Per Serving |
% Daily Value* |
|
Sodium 402 mg |
18% |
|
(as sodium citrate 1,500 mg) | |
|
*Percent Daily Value Based on a 2,000 calorie diet |
|
Serving size: 30 mL (1 Unit Dose) | |
|
Amount Per Serving |
%Daily Value* |
|
Sodium 803 mg |
36% |
|
(as sodium citrate 3,000 mg) | |
|
*Percent Daily Value Based on a 2,000 calorie diet |
HEALTH CLAIM SECTION
Other ingredients: For 1,500 mg-1,002 mg/15 mL Unit Dose - Purified water, citric acid (334 mg/5 mL-1,002 mg/15 mL unit dose), sodium benzoate, flavor, sucralose
For 3,000 mg-2,004 mg/30 mL Unit Dose - Purified water, citric acid (334 mg/5 mL-2,004 mg/30 mL unit dose), sodium benzoate, flavor, sucralose
Use: Use as indicated by a healthcare professional as a dietary supplement. Sodium citrate and citric acid may be beneficial as a systemic alkalizer†
DOSAGE & ADMINISTRATION SECTION
Storage
• store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature]
• preserve in tight, light-resistant containers
• protect from freezing and light
• do not use if lid is torn or broken
Questions or comments? Call-1-844-474-7464
†This statement has not been evaluated by the Food and Drug Administration.
This product is not intended to diagnose, treat, cure, or prevent any disease
Distributed by:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
FG-42 (for 15 mL)
FG-43 (for 30 mL)
Iss.:04/2023
SAFE HANDLING WARNING SECTION
Do not use:
If you are on a sodium restricted diet
Allergic to any of the ingredients
WARNINGS SECTION
PRECAUTIONS SECTION
If pregnant or breast-feeding, ask a doctor before use.
