GOPRELTO
These highlights do not include all the information needed to use GOPRELTO safely and effectively. See full prescribing information for GOPRELTO. GOPRELTO (cocaine hydrochloride) nasal solution, for intranasal use, CII Initial U.S. Approval: 2017
689750b7-8e51-47d9-a428-078f3f6c9dec
HUMAN PRESCRIPTION DRUG LABEL
Aug 8, 2023
Genus Lifesciences Inc.
DUNS: 113290444
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
cocaine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
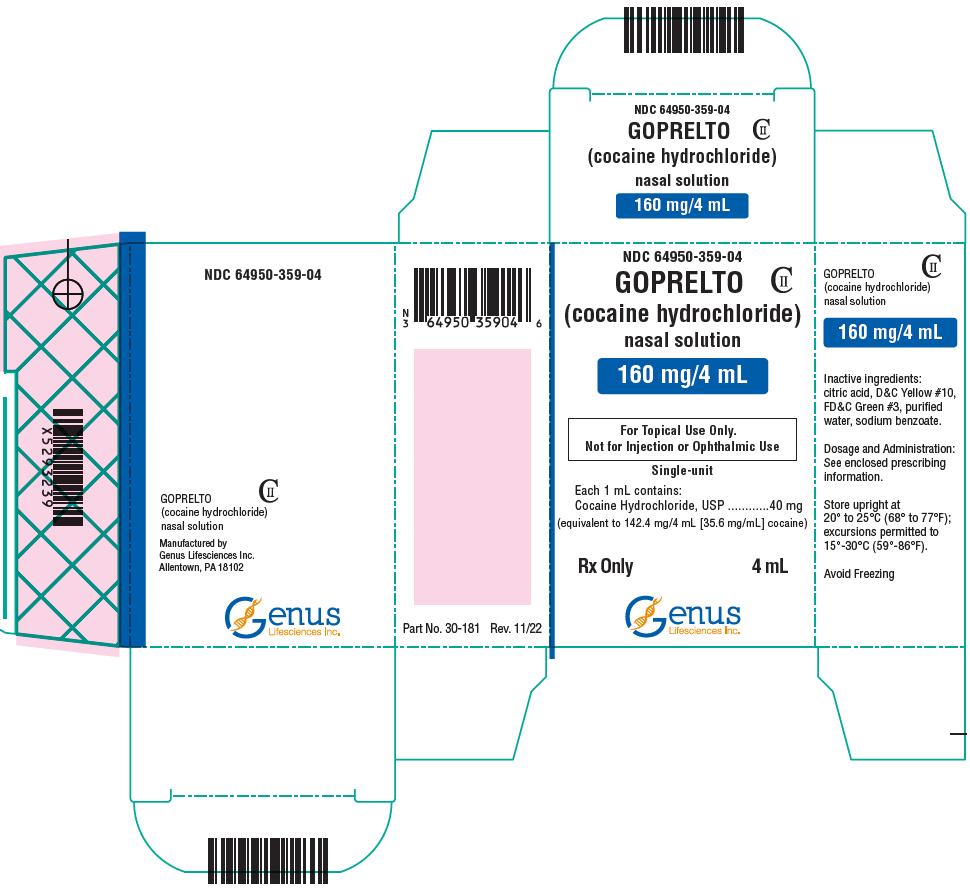
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 4 mL Bottle Carton
NDC 64950-359-04
GOPRELTO
CII
(cocaine hydrochloride)
nasal solution
160 mg/4 mL
For Topical Use Only.
Not for Injection or Ophthalmic Use
Single-unit
Each 1 mL contains:
Cocaine Hydrochloride, USP
40 mg
(equivalent to 142.4 mg/4 mL [35.6 mg/mL] cocaine)
Rx Only
4 mL
Genus
Lifesciences Inc.
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
GOPRELTO has been evaluated in four Phase 1 studies and one Phase 3 study, which included 647 adult subjects who received a single topical intranasal 160 mg dose (four pledgets), of GOPRELTO. The randomized, double-blind, controlled Phase 3 study was conducted in adult patients undergoing diagnostic procedures and surgeries on or through the mucous membranes of the nasal cavities, of which 278 received GOPRELTO (4% solution), 275 received cocaine hydrochloride solution 8%, and 95 received placebo. Safety was evaluated for up to 7 days after dosing.
The most commonly reported adverse reactions (>1 patient) to occur in the Phase 3 study with GOPRELTO (4% solution) were headache and epistaxis. Two adverse reactions of headache were severe (Table 1).
No premature discontinuations due to an adverse event, serious adverse events, or deaths were reported in the Phase 3 clinical study.
Table 1: Common Adverse Reactions with GOPRELTO in >1 Patient|
System Organ Class / Preferred Term |
GOPRELTO 4% |
Cocaine Hydrochloride Solution 8% |
Placebo |
|---|---|---|---|
|
Nervous System Disorders | |||
|
Headache |
7 (3%) |
4 (2%) |
1 (1%) |
|
Respiratory, Thoracic, and Mediastinal Disorders | |||
|
Epistaxis |
3 (1%) |
2 (1%) |
0 |
|
Psychiatric Disorders | |||
|
Anxiety |
0 |
2 (1%) |
0 |
The most common adverse reactions (>0.5%) occurring in patients treated with GOPRELTO (cocaine hydrochloride) nasal solution 4% were headache and epistaxis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pharm-Olam at 1-866-511-6754 or FDA at 1-800-FDA-1088 or****www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Disulfiram
Published literature reported that disulfiram treatment increased plasma cocaine exposure, including both AUC and Cmax, by several fold after acute intranasal cocaine administration. Other literature reported that co- administration of disulfiram increased AUC of plasma cocaine by several fold after intravenous cocaine administration [see Clinical Pharmacology (12.3)].
Avoid using GOPRELTO in patients taking disulfiram. Consider using other local anesthetic agents.
7.2 Epinephrine, Phenylephrine
There are reports in the published literature of myocardial ischemia, myocardial infarction, and ventricular arrhythmias after concomitant administration of topical intranasal cocaine with epinephrine and phenylephrine during nasal and sinus surgery.
Avoid use of additional vasoconstrictor agents such as epinephrine and phenylephrine with GOPRELTO during nasal and sinus surgery. If concomitant use is unavoidable, prolonged vital sign and ECG monitoring may be required [see Warnings and Precautions (5.3)].
7.3 Inhibitors of plasma cholinesterase (pseudocholinesterase)
Cocaine has been described in literature to be primarily metabolized and inactivated by non-enzymatic ester hydrolysis and hepatic carboxylesterase, and also by plasma cholinesterase, hepatic carboxylesterase, and CYP3A4 [see Clinical Pharmacology (12.3)]. The pharmacokinetics of GOPRELTO in patients with reduced plasma cholinesterase activity has not been studied.
Plasma cholinesterase activity may be decreased by chronic administration of certain monoamine oxidase inhibitors, oral contraceptives, or glucocorticoids. It may also be diminished by administration of irreversible plasma cholinesterase inhibitors such as echothiophate, organophosphate insecticides, and certain antineoplastic agents. Patients with reduced plasma cholinesterase (pseudocholinesterase) activity may have reduced clearance and increased exposure of plasma cocaine after administration of GOPRELTO.
Since cocaine is metabolized by multiple enzymes, the effect of reduced plasma cholinesterase activity on cocaine exposure may be limited. No dosage adjustment of GOPRELTO is needed in patients with reduced plasma cholinesterase. Monitor patients with reduced plasma cholinesterase activity for adverse reactions such as headache, epistaxis, and clinically-relevant increases in heart rate or blood pressure.
Disulfiram: Increases plasma cocaine exposure. Avoid using GOPRELTO in patients taking disulfiram. (7)
Epinephrine, Phenylephrine: There have been reports of myocardial ischemia, myocardial infarction, and ventricular arrhythmias with concomitant use during nasal surgery. Avoid use of additional vasoconstrictor agents with GOPRELTO. If concomitant use is unavoidable, prolonged vital sign and ECG monitoring may be required. (5.3, 7)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- GOPRELTO is for intranasal use only.
- Do not apply GOPRELTO to damaged nasal mucosa.
2.2 Dosing Recommendation for Adults
The recommended dose of GOPRELTO is two soaked cottonoid pledgets placed in each nasal cavity, equivalent to 40 mg cocaine hydrochloride per pledget, for a total dose of 160 mg for four pledgets.
The total dose for any one procedure or surgery should not exceed 160 mg, or 3 mg/kg, cocaine hydrochloride.
The recommended size of cottonoid pledgets for use with GOPRELTO measure 1.3 cm × 4 cm (sold separately).
2.3 Preparation and Administration of GOPRELTO Pledgets
Pour the full contents of one 4 mL (160 mg) bottle of GOPRELTO into a small container. Soak four cottonoid pledgets until the solution is fully absorbed.
Following soaking, place two pledgets in each nasal cavity against the septum.
Leave pledgets in place for up to twenty minutes. Remove pledgets and continue with the procedure. Discard pledgets and dispose of any unused portion of solution in accordance with institutional procedures for CII products.
- For intranasal use only. (2.1)
- Recommended dose: two pledgets, each containing 40 mg of cocaine hydrochloride, applied to each nasal cavity. (2.2)
- Do not apply to damaged nasal mucosa. (2.1)
- Preparation and Application:
- In a small container, soak four pledgets in the full contents (4 mL) of one bottle of GOPRELTO until the solution is fully absorbed. Each pledget absorbs 1 mL of solution, equivalent to 40 mg cocaine hydrochloride. (2.2, 2.3)
- Following soaking, place two pledgets in each nasal cavity against the septum. (2.3)
- Leave pledgets in place for up to 20 minutes. (2.3)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of cocaine have not been conducted.
Mutagenesis
In published studies, cocaine was genotoxic in the in vitro chromosomal aberration assay, the in vitro sister chromatid exchange assay, the in vitro micronucleus assay, and the in vitro hypoxanthine-guanine phosphoribosyltransferase (hgprt) assay. Cocaine was equivocal in a published in vivo micronucleus assay and the in vivo comet assay (liver). Cocaine was not mutagenic in the in vitro bacterial reverse mutation assay (Ames assay).
Impairment of Fertility
No adverse effects on fertility or early embryonic development were reported in a study where male rats were administered up to 20 mg/kg cocaine hydrochloride via subcutaneous injection for 28 days prior to mating and female rats were treated with the same dose for 14 days prior to mating through Gestation Day 7. The 20 mg/kg/dose resulted in AUC exposures that were 31 times (males) and 47 times (females) the adult human AUC following administration of pledgets containing 160 mg of cocaine.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
A double-blind, multicenter, single-dose, placebo- and dose-controlled, parallel-group study was conducted in 648 subjects undergoing diagnostic procedures and surgeries on or through the mucous membranes of the nasal cavities. Subjects were randomized to receive GOPRELTO (n=278), cocaine hydrochloride solution 8% (n=275), to explore the dosing range, or placebo (n=95). Nasal endoscopy, nasal laryngoscopy, nasopharyngeal laryngoscopy, and nasal debridement comprised 88% of all procedures performed in the GOPRELTO group and 85% of all procedures performed in the placebo group. All subjects completed the diagnostic or surgical procedure.
In the GOPRELTO group, two 40 mg pledgets were applied to the septum in each nasal cavity (160 mg cocaine hydrochloride total dose) and left in place for up to 20 minutes. Similarly, pledgets were applied in the placebo group. Topical anesthesia was assessed using the visual numeric rating scale (VNRS) during a von Frey Filament test prior to the diagnostic procedure or surgery. After subject-reported pain scores were collected, the blind to placebo was broken and placebo subjects were provided the option of receiving anesthesia. The primary efficacy endpoint was analgesic success, defined in the GOPRELTO group as a subject-reported pain score of 0 (no pain) on the VNRS during the von Frey Filament test, and no additional anesthetic or analgesic medication administration during the diagnostic procedure or surgery. Analgesic success was defined in the placebo group as a subject-reported pain score of 0 on the VNRS during the von Frey Filament test. Subjects did not receive supplemental intravenous sedation or general anesthesia during the study.
Table 2 provides the efficacy results for the primary endpoint of analgesic success showing a significant difference in the analgesic success rate between placebo and GOPRELTO.
Table 2: Analgesic Success|
Event |
GOPRELTO |
Placebo |
|---|---|---|
|
Success |
215 (77%) |
14 (15%) |
|
Failure |
63 (23%) |
81 (85%) |
Of the 63 (23%) failures in the GOPRELTO group, 4 subjects requested additional anesthetic medication. Of these 4 subjects, 1 subject reported 0 on the VNRS during the von Frey Filament test. Of the 81 (85%) failures in the placebo group, 50 subjects required additional anesthetic medication.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
GOPRELTO (cocaine hydrochloride) nasal solution is a clear, green colored liquid available as one dosage strength:
160 mg/4 mL (40 mg/mL or 4%) cocaine hydrochloride, equivalent to 142.4 mg/4mL (35.6 mg/mL) cocaine
NDC # 64950-359-04: Single-unit 4 mL bottle
Store upright at 20° to 25°C (68° to 77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP, Controlled Room Temperature (CRT)]. Avoid freezing.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Potential for Abuse and Dependence
Advise patients that GOPRELTO is a controlled substance and it can be abused and lead to dependence [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9)].
Toxicology Screening
Advise patients that the cocaine hydrochloride in GOPRELTO may be detected in plasma for up to one week after administration. Cocaine hydrochloride and its metabolites may be detected in urine toxicology screening for longer than one week after administration. [see Warnings and Precautions (5.4)].
Seizures
Advise patients that GOPRELTO may lower the seizure threshold. Patients should be monitored for development of seizures. [see Warnings and Precautions (5.2)].
Blood Pressure and Heart Rate Increase
Advise patients that GOPRELTO can cause increases in blood pressure and heart rate and should be avoided in patients with recent or active history of uncontrolled hypertension, unstable angina, myocardial infarction, coronary artery disease, or congestive heart failure [see Warnings and Precautions (5.3)].
Headache and/or Epistaxis
Inform patients that headache and/or epistaxis are the most frequently experienced side effects that should resolve without treatment. Instruct patients to contact their health care professional if these symptoms persist [see Adverse Reactions (6)].
Pregnancy
Inform female patients of reproductive potential that GOPRELTO may cause fetal harm and to inform their prescriber of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise a nursing woman that breastfeeding is not recommended during treatment with GOPRELTO and to pump and discard breastmilk for 48 hours after administration of GOPRELTO nasal solution [see Use in Specific Populations (8.2)].
