KOSHER MEDS antacid
7783d6a2-56e3-4542-ad1d-60680e03532b
HUMAN OTC DRUG LABEL
Jun 23, 2025
Mollec Inc
DUNS: 040088196
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Calcium carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product label


INDICATIONS & USAGE SECTION
Uses
relieves
- heartburn
- sour stomach
- acid indigestion
- upset stomach associated with these symptoms
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Calcium carbonate 500 mg (in each chewable tablet)
OTC - PURPOSE SECTION
Purpose
Antacid
WARNINGS SECTION
Warnings
Ask a doctor or pharmacist before use****if you arepresently taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- do not take more than 8 tablets in 24 hours
- if pregnant do not take more than 5 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks except under the advice and supervision of a doctor
Keep out of reach of children.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
DOSAGE & ADMINISTRATION SECTION
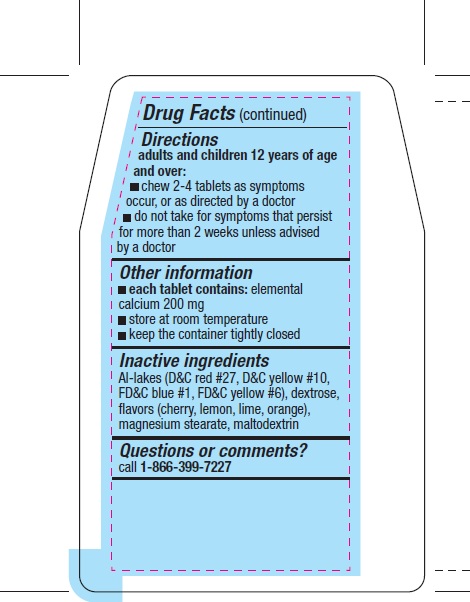
Directions
adults and children 12 years of age and over:
- chew 2-4 tablets as symptoms occur, or as directed by a doctor
- do not take for symptoms that persist for more than 2 weeks unless advised by a doctor
SPL UNCLASSIFIED SECTION
Other information
*each tablet contains: elemental calcium 200 mg
- store at room temperature
- keep the container tightly closed
INACTIVE INGREDIENT SECTION
Inactive ingredients
Al-lakes (D&C red #27, D&C yellow #10, FD&C blue #1, FD&C yellow #6), dextrose, flavors (cherry, lemon, lime, orange), magnesium stearate, maltodextrin
OTC - QUESTIONS SECTION
Questions or comments?
call1-866-399-7227
