Chlorpromazine Hydrochloride
Chlorpromazine Hydrochloride Injection, USP
d37a4c21-b503-4791-a4d0-2ccc8891d88e
HUMAN PRESCRIPTION DRUG LABEL
Mar 14, 2024
Zydus Lifesciences Limited
DUNS: 918596198
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Chlorpromazine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Chlorpromazine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
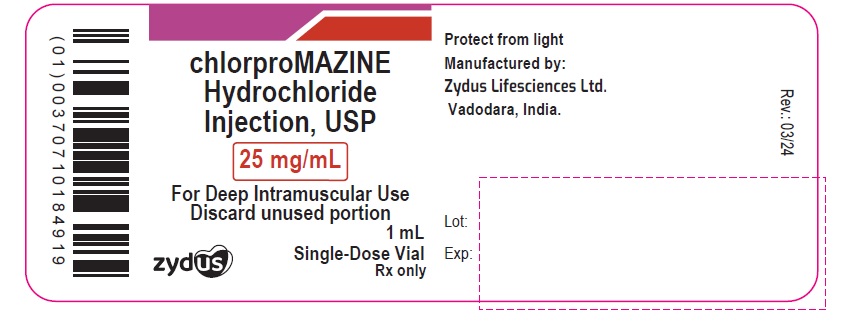
NDC 70771-1778-1
chlorproMAZINE Hydrochloride Injection, USP
25 mg/mL
For Deep Intramuscular Use
Discard unused portion
1 mL Single-Dose Vial
Rx only
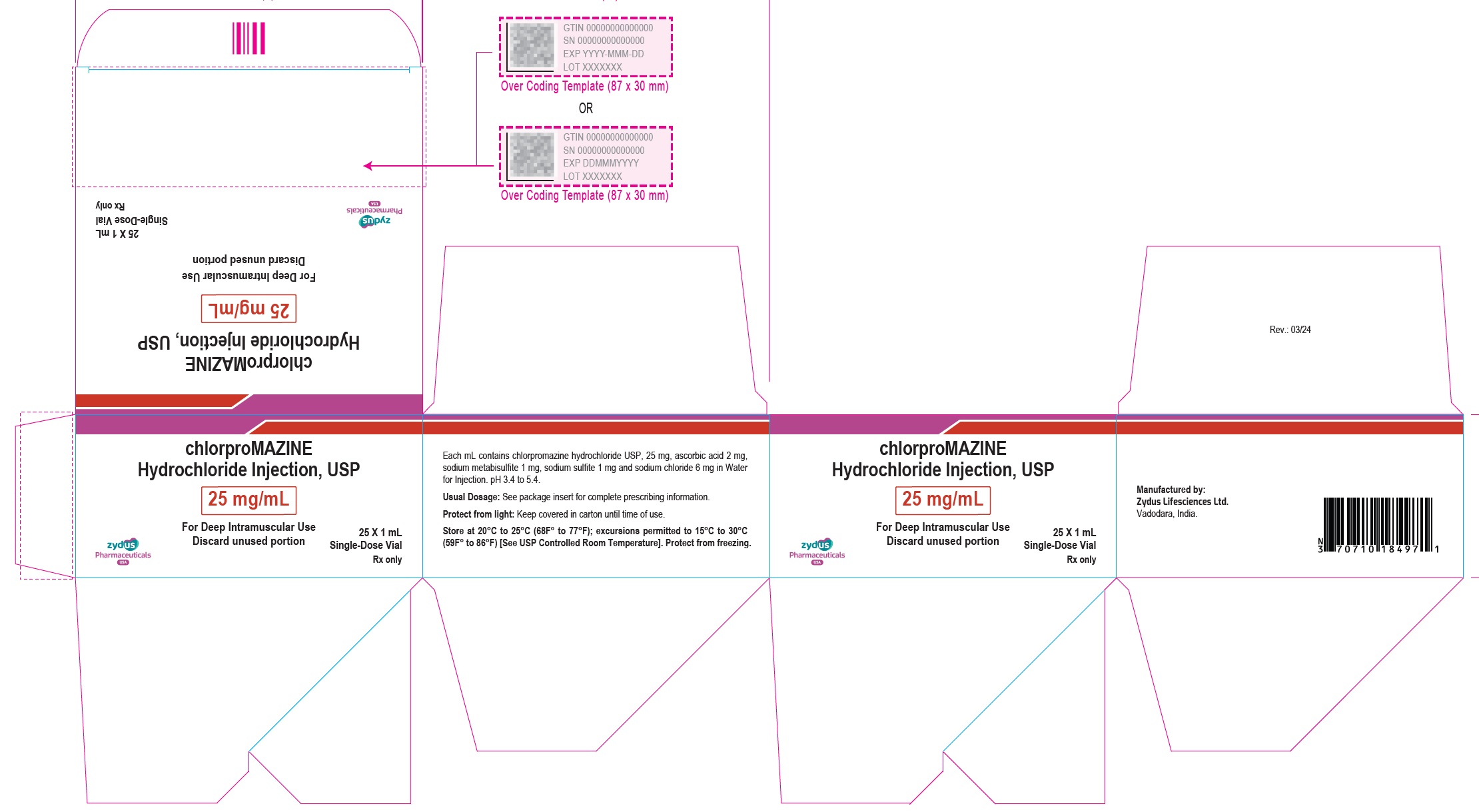
NDC 70771-1778-7
chlorproMAZINE Hydrochloride Injection, USP
25 mg/mL
For Deep Intramuscular Use
Discard unused portion
25 x 1 mL Single-Dose Vial
Rx only