Tolnaftate Cream 1% ANTIFUNGAL
Tolnaftate Cream 1% ANTIFUNGAL CREAM
96a40ccd-17f7-4e4e-98da-207802fa2b2d
HUMAN OTC DRUG LABEL
May 15, 2025
Trifecta Pharmaceuticals Usa Llc
DUNS: 079424163
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Tolnaftate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
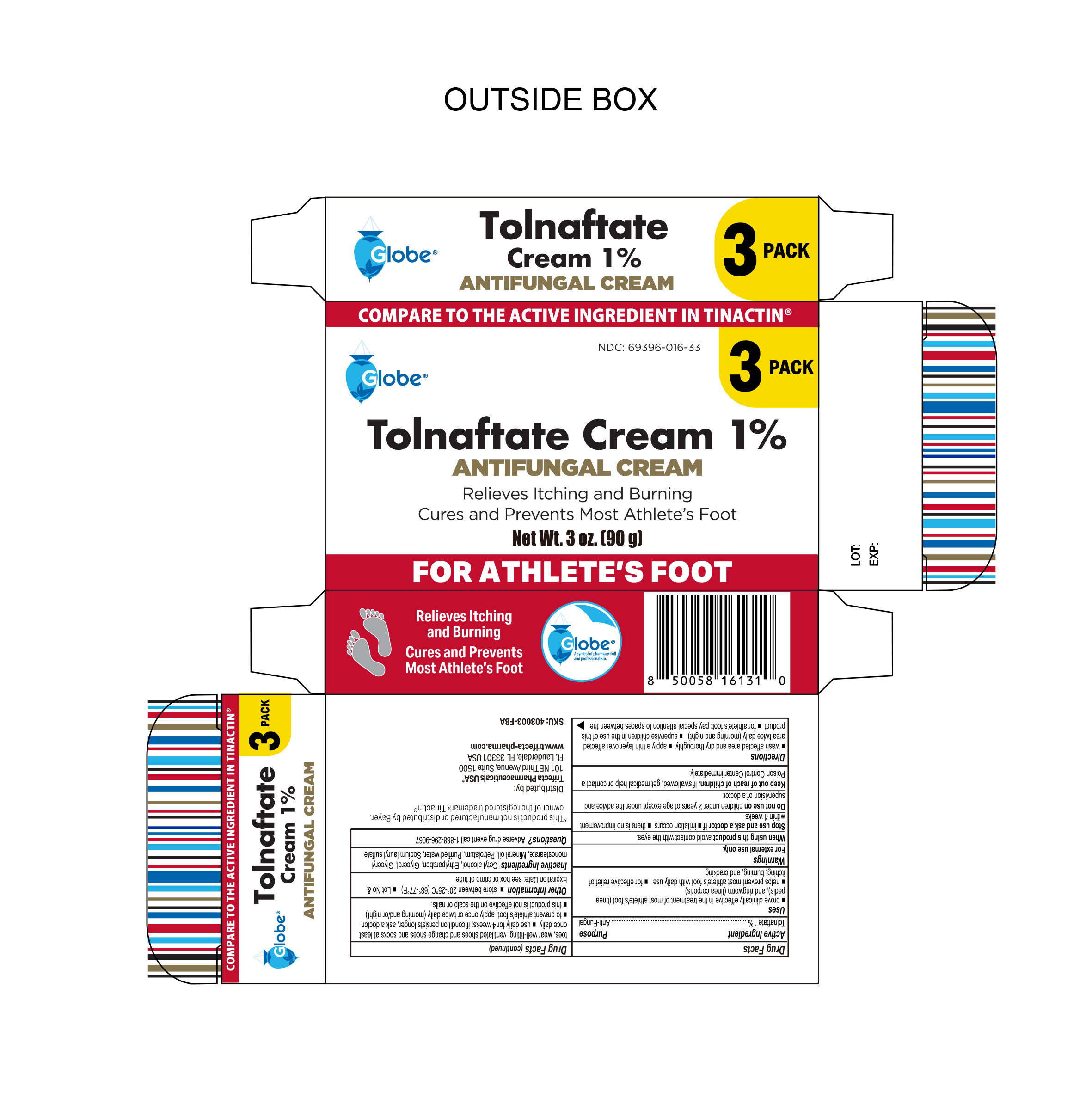
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
INDICATIONS & USAGE SECTION
Uses
● proven clinically effective in the treatment of most athlete's foot (tinea pedis), and ringworm (tinea corporis) ● helps prevent most athlete's foot with daily use ● for effective relief of itching, burning and cracking.
SPL UNCLASSIFIED SECTION
100% GUARANTEED
Cures and Prevents Most Athlete's Foot
Distributed by:
Trifecta Pharmaceuticals USA™
101 NE Third Avenue, Suite 1500
Ft. Lauderdale, FL 33301 USA
Product of PRC
www.trifecta-pharma.com
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Tolnaftate 1%
OTC - PURPOSE SECTION
Purpose
Anti-Fungal
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
WARNINGS SECTION
Warnings
For external use only.
When using this product avoid contact with the eyes.
Stop use and ask a healthcare professional if ● irritation occurs ● there is no improvement within 4 weeks improvement
Do not use on children under 2 years of age except under the advice and supervision of a healthcare professional.
DOSAGE & ADMINISTRATION SECTION
Directions
● wash affected area and dry thoroughly
● apply a thin layer over affected area twice daily (morning and night)
● supervise children in the use of this product
● for athlete’s foot: pay special attention to spaces between the toes, wear well-fitting ventilated shoes and change shoes and socks at least once daily.
● use daily for 4 weeks; if conditions persists longer, ask a healthcare professional.
● to prevent athlete's foot, apply once or twice daily (morning and/or night)
● this product is not effective on the scalp or nails.
INACTIVE INGREDIENT SECTION
Inactive ingredients
Cetostearyl alcohol, ethylparaben, glycerol, light mineral oil, monostearin, petrolatum, purified water, sodium dodecyl sulfate
STORAGE AND HANDLING SECTION
Other information
● store between 20° to 25°C ( 68° to 77°F) ● Lot No & Expiration Date: See box or crimp of tube.
