PROSTAT FIRST AID Burn Ease, 0.9g
58228-3867-1 PS-3866 Burn Ease 0.9g
dc704403-e2aa-47f7-a7f4-f6afe1547ca8
HUMAN OTC DRUG LABEL
Oct 1, 2025
ProStat First Aid LLC
DUNS: 061263699
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
LIDOCAINE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
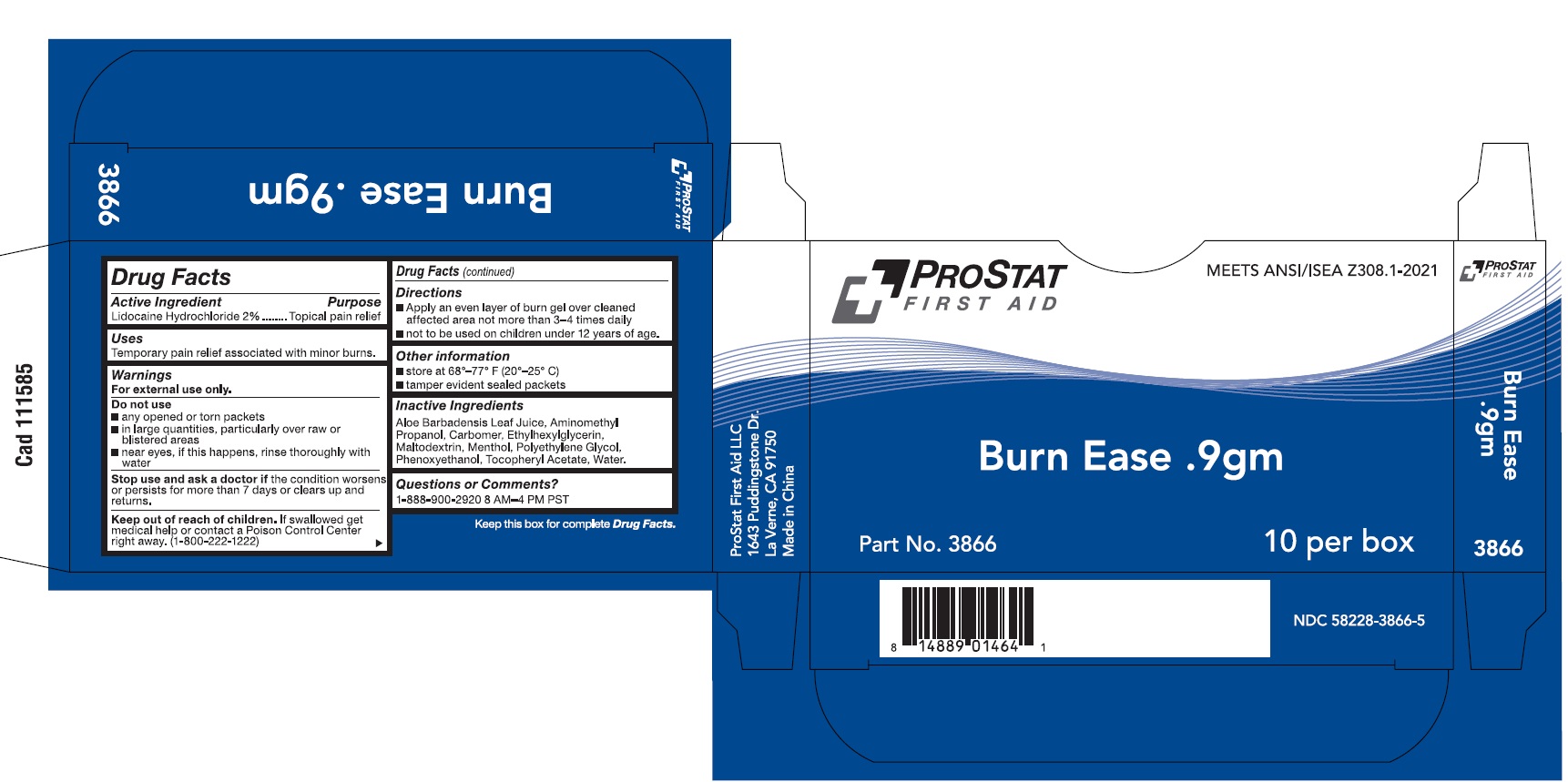
INDICATIONS & USAGE SECTION
Use(s)
Temporary pain relief associated with minor burns
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Aminomethyl propanol, Carbomer, Ethylhexylglycerin, Maltodextrin, Menthol, Polyethylene glycol, Phenoxyethanol, Tocopheryl Acetate, Water
OTC - QUESTIONS SECTION
Questions?
1-888-900-2920 8AM - 4PM PST
OTHER SAFETY INFORMATION
Other Information
• store at 68-77F (20-25C)
- tamper evident sealed packets
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Lidocaine Hydrochloride 2%
OTC - PURPOSE SECTION
Purpose
Topical Pain Relief
WARNINGS SECTION
Warnings
For External Use Only
Do not use
• any opened or torn packets
• in large quantities, particularly over raw or blistered areas
- Near eyes. If this happens, rinse thoroughly with water
• Avoid contact with the eyes
• Do not bandage tightly
Stop use and ask a doctor if
• Condition worsens or persists for more than 7 days or clears up and returns
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
DOSAGE & ADMINISTRATION SECTION
Directions
Apply an even layer of burn gel over cleaned affected area not more than 3-4 times daily
Not to be used on children under 12 years of age
