Vicks VapoCool
VICKS VapoCOOL™ SEVERE + Intense Pain Relief Drops
38cd83db-47ca-e320-e063-6394a90aba41
HUMAN OTC DRUG LABEL
Jun 30, 2025
The Procter & Gamble Manufacturing Company
DUNS: 004238200
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Benzocaine and Menthol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
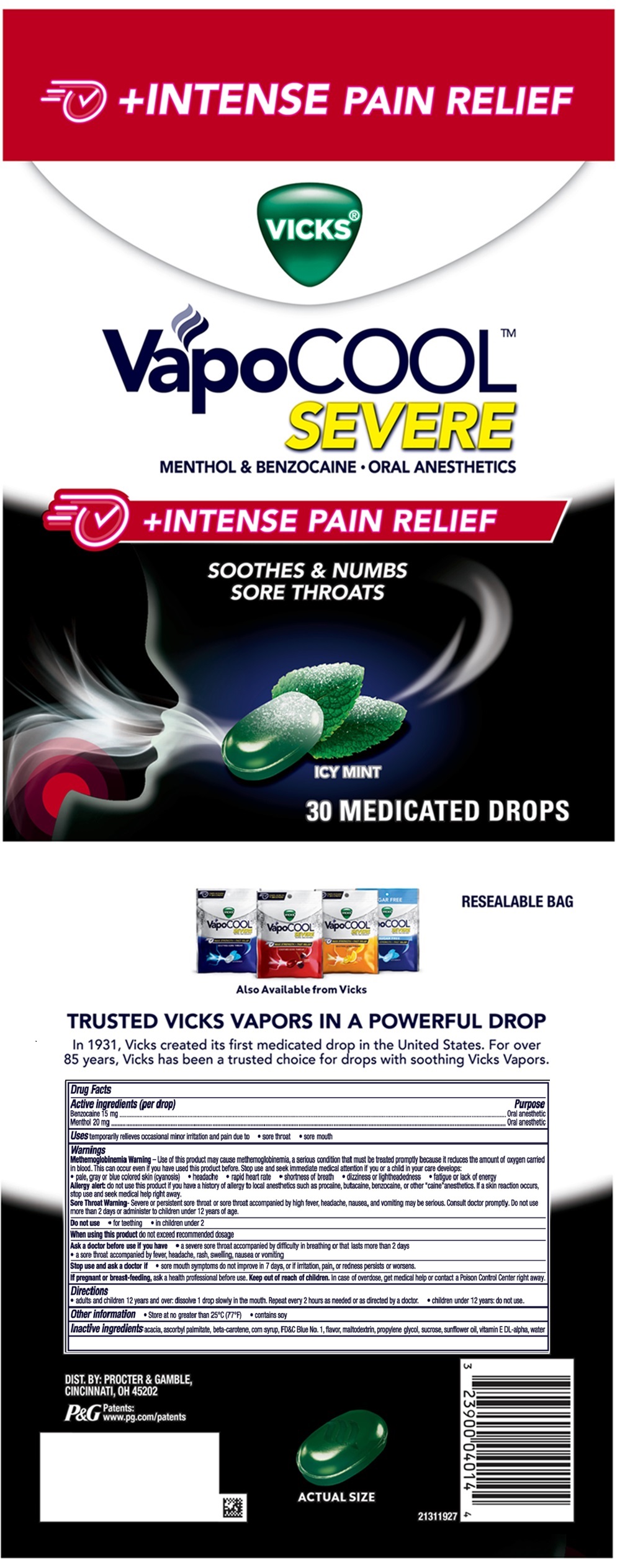
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30 MEDICATED DROPS
VICKS ®
VapoCOOL
SEVERE
MENTHOL & BENZOCAINE - ORAL ANESTHETICS
+INTENSE PAIN RELIEF
SOOTHES & NUMBS
SORE THROATS
ICY MINT
30 MEDICATED DROPS
INDICATIONS & USAGE SECTION
Uses
temporarily relieves occasional minor irritation and pain due to
- sore throat
- sore mouth
SPL UNCLASSIFIED SECTION
DIST. BY PROCTER & GAMBLE,
** CINCINNATI OH 45202**
OTC - ACTIVE INGREDIENT SECTION
Active ingredients (per drop)
Benzocaine 15 mg
Menthol 20 mg
Purpose
Oral anesthetic
Oral anesthetic
STORAGE AND HANDLING SECTION
Other information
- Store at no greater than 25°C (77°F)
- contains soy
INACTIVE INGREDIENT SECTION
Inactive ingredients
acacia, ascorbyl palmiltate, beta-carotene, corn syrup, FD&C Blue No. 1, flavor, maltodextrin, propylene glycol, sucrose, sunflower oil, vitamin E DL- alpha, water
OTC - QUESTIONS SECTION
Questions?
1-800-707-1709
WARNINGS SECTION
Warnings
Methemoglobinemia Warning–
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
OTC - DO NOT USE SECTION
Do not use
- for teething
- in children under 2
OTC - ASK DOCTOR SECTION
Ask a doctor before use if you have
- a severe sore throat accompanied by difficulty in breathing or that lasts more than 2 days
- a sore throat accompanied by fever, headache, rash, swelling, nausea or vomiting
OTC - STOP USE SECTION
Stop use and ask a doctor if
- sore mouth symptoms do not improve in 7 days, or if irritation, pain, or redness persists or worsens
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
OVERDOSAGE SECTION
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 12 years and over: dissolve 1 drop slowly in the mouth. Repeat every 2 hours as needed or as directed by a doctor.
- children under 12 years: do not use.
