TheraCare Roll-on Menthol 4% Pain Relief
TheraCare Roll-on Menthol 4% Pain Relief Gel
fa121e5f-94b5-4c1d-9c4e-85bb8ede3945
HUMAN OTC DRUG LABEL
Sep 22, 2025
Veridian Healthcare
DUNS: 830437997
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Menthol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (20)
Drug Labeling Information
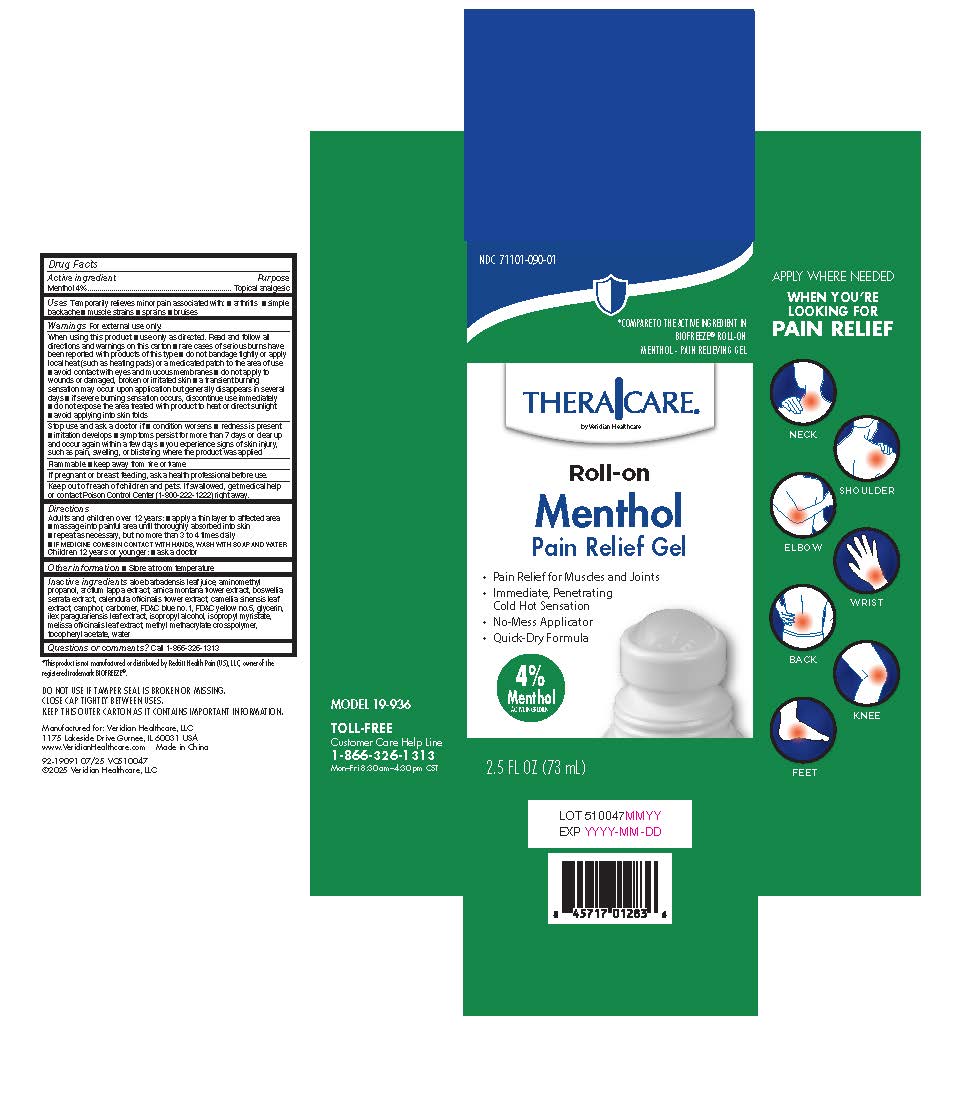
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
INDICATIONS & USAGE SECTION
Uses
Uses Temporarily relieves minor pain associated with: ■ arthritis ■ simple backache ■ muscle strains ■ sprains ■ bruises
SPL UNCLASSIFIED SECTION
TheraCare Roll-on Menthol 4% Pain Relief Gel
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Menthol 4%
OTC - PURPOSE SECTION
Purpose
Topical analgesic
WARNINGS SECTION
Warnings
For external use only
When using this product
When using this product
■ use only as directed. Read and follow all directions and warnings on this carton
■ rare cases of serious burns have been reported with products of this type
■ do not bandage tightly or apply local heat (such as heating pads) or a
medicated patch to the area of use
■ avoid contact with eyes and mucous membranes
■ do not apply to wounds or damaged, broken or irritated skin
■ a transient burning sensation may occur upon application but generally disappears in several days
■ if severe burning sensation occurs, discontinue use immediately
■ do not expose the area treated with the product to heat or direct sunlight
■ avoid applying into skin folds
Stop use and ask a doctor if
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
- you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
Flammable■ Keep away from fire or flame
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
DOSAGE & ADMINISTRATION SECTION
Directions
Adults and children over 12 years:■ apply a thin layer to affected area
■ massage into painful area until thoroughly absorbed into skin
■ repeat as necessary, but no more than 3 to 4 times daily
■IF MEDICINE COMES IN CONTACT WITH HANDS, WASH WITH SOAP AND WATER
Children 12 years or younger:■ ask a doctor
INACTIVE INGREDIENT SECTION
Inactive ingredients
Inactive ingredients aloe barbadensis leaf juice, aminomethyl propanol, arctium lappa extract, arnica montana flower extract, boswellia serrata extract, calendula officinalis flower extract, camellia sinensis leaf extract, camphor, carbomer, FD&C blue no.1, FD&C yellow no.5, glycerin, ilex paraguariensis leaf extract, isopropyl alcohol, isopropyl myristate, melissa officinalis leaf extract, methyl methacrylate crosspolymer, tocopheryl acetate, water
OTC - QUESTIONS SECTION
Questions or comments
Call 1-866-326-1313
