Triamcinolone Acetonide
Triamcinolone Acetonide 0.05% in Absorbase (Triamcinolone Acetonide Ointment, USP) Proprietary Hydrous Emulsified Base Rx Only
5067e7da-3eed-4d6a-b870-47a35c3ded81
HUMAN PRESCRIPTION DRUG LABEL
Jan 17, 2023
CMP Pharma, Inc.
DUNS: 005224175
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Triamcinolone Acetonide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Topical corticosteroids, such as Triamcinolone Acetonide 0.05% in Absorbase® (Triamcinolone Acetonide Ointment, USP), constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Each gram of Triamcinolone Acetonide 0.05% in Absorbase® (Triamcinolone Acetonide Ointment, USP) contains 0.5 mg of Triamcinolone Acetonide USP in a water-in-oil emulsion composed of Light Mineral Oil NF, Purified Water USP, White Petrolatum USP, Heavy Mineral Oil USP, Mineral Wax, and Lanolin Alcohols NF. The white ointment is for topical use only.
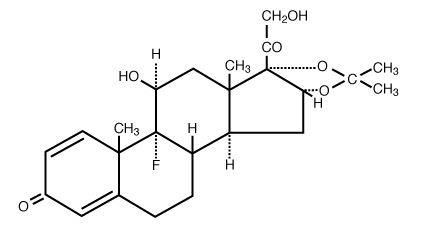
Triamcinolone Acetonide has the molecular formula of C 24H 31FO 6 and is designated chemically as Pregna-1,4-diene-3, 20-dione, 9-fluoro-11, 21-dihydroxy-16, 17-[(1-methylethylidene)bis(oxy)]-, (11β, 16α)-. It has a molecular weight of 434.50 and the following structural formula:
HOW SUPPLIED SECTION
HOW SUPPLIED
Triamcinolone Acetonide 0.05% in Absorbase® (Triamcinolone Acetonide Ointment, USP) is supplied in 110 g jars (NDC 46287-010-11).
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
To report SUSPECTED ADVERSE REACTIONS, contact CMP Pharma at 1-844-321-1443 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
DISPENSE IN A WELL-CLOSED CONTAINER.
Rx Only
For external use only. Not for ophthalmic use.
