Tagrid Antifungal
Initial Drug Listing-Tagrid Antifungal Soap Kit
3663e054-9411-358a-e063-6394a90af6b4
HUMAN OTC DRUG LABEL
Jun 9, 2025
TAGRID LLC
DUNS: 118778969
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Tolnaftate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
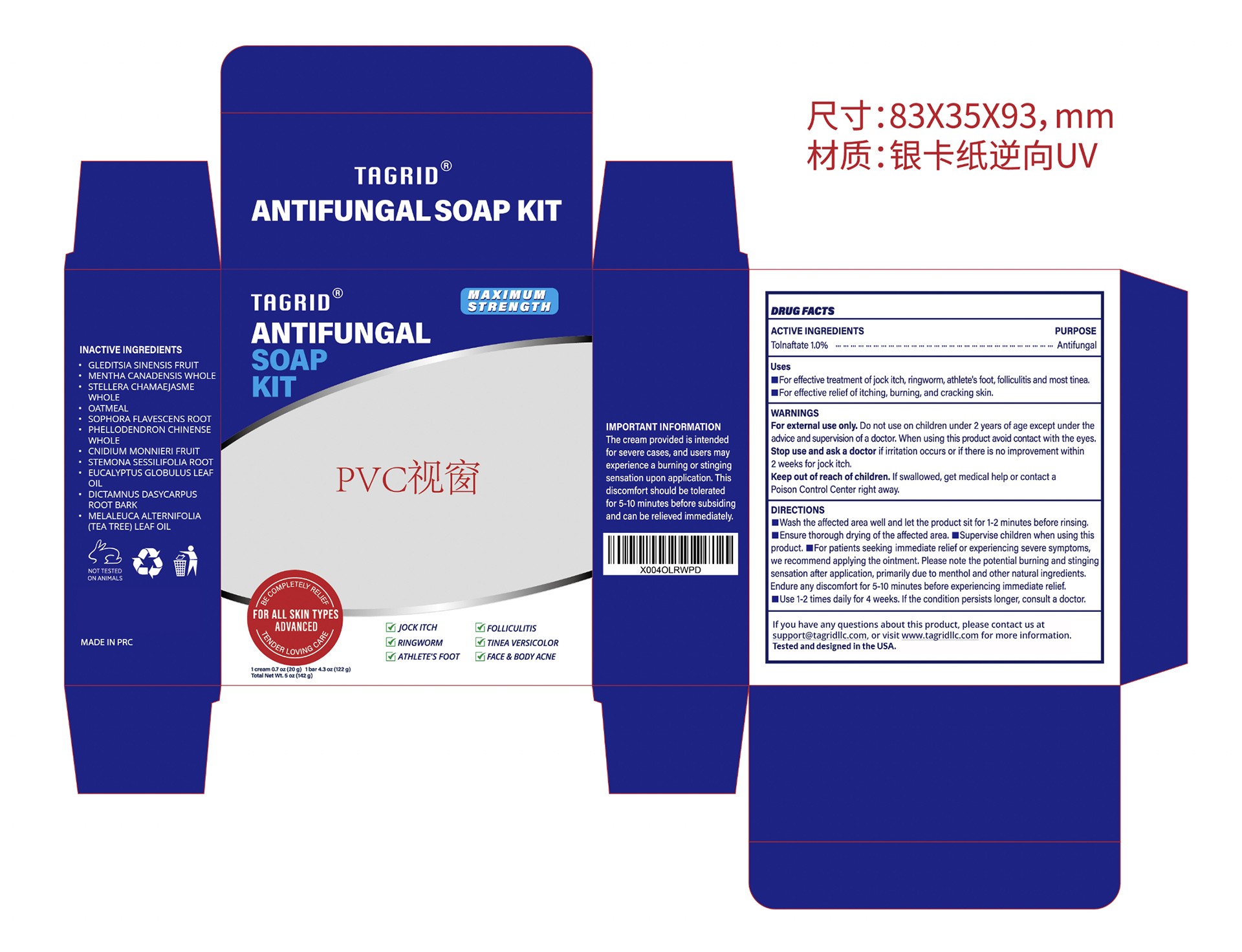
INDICATIONS & USAGE SECTION
Uses
For effective treatment of jock itch, ringworm, athlete's foot, folliculitnost
tinea
For effective relief of itching, burning, and cracking sking skin.
INACTIVE INGREDIENT SECTION
GLEDITSIA SINENSIS FRUIT
MENTHA CANADENSIS WHOLE
STELLERA CHAMAEJASME WHOLE
OATMEAL
SOPHORA FLAVESCENS ROOT
PHELLODENDRON CHINENSE WHOLE
CNIDIUM MONNIERI FRUIT
STEMONA SESSILIFOLIA ROOT
EUCALYPTUS GLOBULUS LEAF OIL
DICTAMNUS DASYCARPUS ROOT BARK
MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL
OTC - ACTIVE INGREDIENT SECTION
Tolnaftate
OTC - PURPOSE SECTION
WARNINGS SECTION
For external use only
Do not use on children under 2 years of age exceptunder the advice and supervision of a doctor.
When using this product avoid contact with the eyes.
Stop use and ask a doctor if irritation occurs or if there is no improvement within 2weeks for jock itch.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
DIRECTIONS
Wash the affected area well and let the product sit for 1-2 minutes before
rising
Ensure thorough drying of the affected area.
Supervise children when using this product.
For patients seeking immediate relief or experiencing severe symptoms, we
recommend applying the ointment. Please note the potential burning and
stinging sensation after application, primarily due to menthol and other
natural ingredients.
Endure any discomfort for 5-10 minutes before experiencing immediate relief.
Use 1-2 times daily for 4 weeks. If the condition persists longer, consult a
doctor.
OTHER SAFETY INFORMATION
The cream provided is intended for severe cases, and users may experience a burning or stinging sensation upon application. This discomfort should be tolerated for 5-10 minutes before subsiding and can be relieved immediately.
OTC - QUESTIONS SECTION
If you have any questions about this product, please contact us at
support@tagridllc.com, or visit www.tagridllc.com for more information.
Tested and designed in the USA.
