Manufacturing Establishments1
FDA-registered manufacturing facilities and establishments involved in the production, packaging, or distribution of this drug product.
Neilmed Pharmaceuticals Inc.
799295915
Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Glycerin Laxative
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
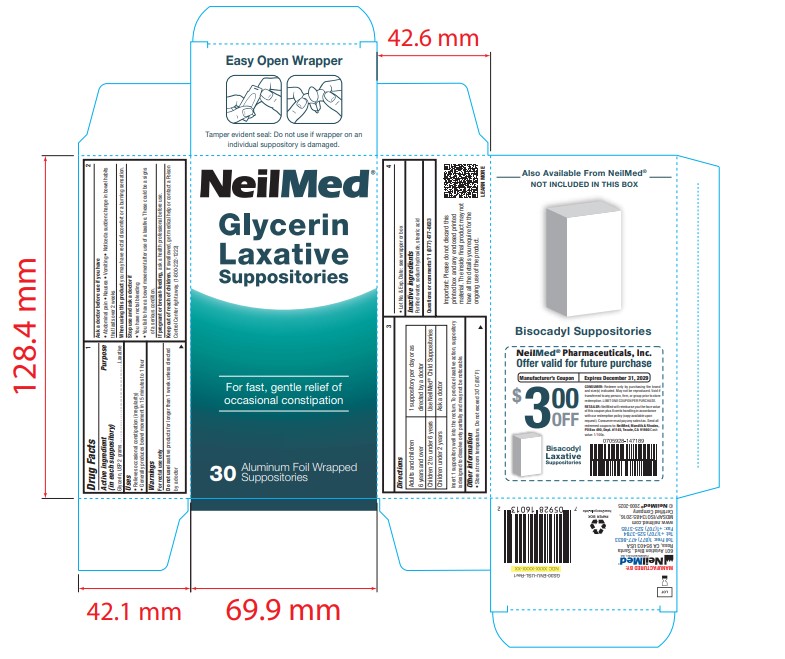 Package Label
Package Label
INDICATIONS & USAGE SECTION
Uses
• Relieves occasional constipation (irregularity)
• Generally produces bowel movement in 15 minutes to 1 hour
DOSAGE & ADMINISTRATION SECTION
Directions
|
Adults and children 6 years of age and over |
1 suppository per day or as directed by a doctor. |
|
Children 2 to under 6 years |
Use Neilmed Child Suppositories |
|
Children under 2 years |
Ask a doctor |
Insert 1 suppository well into the rectum. To produce laxative action, suppository is designed to dissolve only partially and may not be noticeable.
WARNINGS SECTION
Warnings
For rectal use only
SAFE HANDLING WARNING SECTION
Important:
Please do not discard this printed box and any enclosed printed material. The inside final product may not have all the details you require for the ongoing use of the product.
STORAGE AND HANDLING SECTION
Other Information
- Store at room temperature. Do not exceed 30˚ C (86˚ F)
- Lot No. & Exp. Date: see wrapper or box
OTC - ACTIVE INGREDIENT SECTION
Drug Facts: Active Ingredient
Active ingredient (in each suppository)
Glycerin, USP 2 grams
OTC - PURPOSE SECTION
Drug Facts
Active ingredient (in each suppository) ...............Purpose
Glycerin, USP 2 grams ...................................... Laxative
OTC - ASK DOCTOR SECTION
Ask a doctor before use if you have
• Abdominal pain • Nausea • Vomiting • Noticed a sudden change in bowel habits that lasts over 2 weeks
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
OTC - DO NOT USE SECTION
Do not use
laxative products for longer than 1 week unless directed by a doctor
OTC - STOP USE SECTION
Stop use and ask a doctor if
- You have rectal bleeding
- You fail to have a bowel movement after use of a laxative. These could be a signs of a serious condition.
OTC - WHEN USING SECTION
When using the product
you may have rectal discomfort or a burning sensation.
OTC - QUESTIONS SECTION
Questions?
Questions or comments? 1 (877) 477-8633
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding,
ask a health professional before use.
INACTIVE INGREDIENT SECTION
Inactive ingredients
Purified water, sodium hydroxide, stearic acid
