SIMETHICONE INFANT
SIMETHICONE INFANT GAS RELIEF DROPS fast instant gas relief
9b0fde90-35ae-498e-99cc-d120f1a87182
HUMAN OTC DRUG LABEL
Jul 7, 2025
AARNA USA Inc.
DUNS: 118515992
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Simethicone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

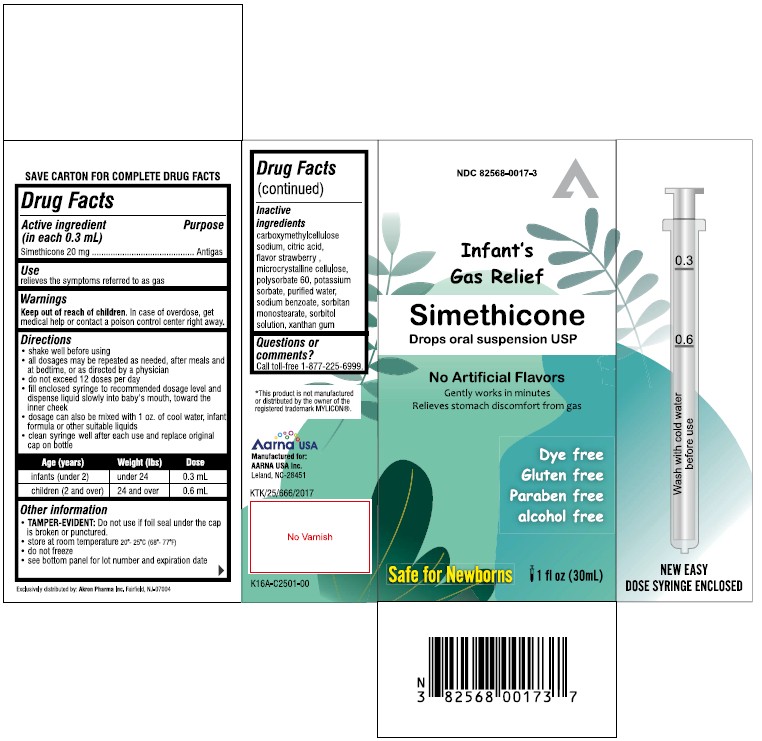
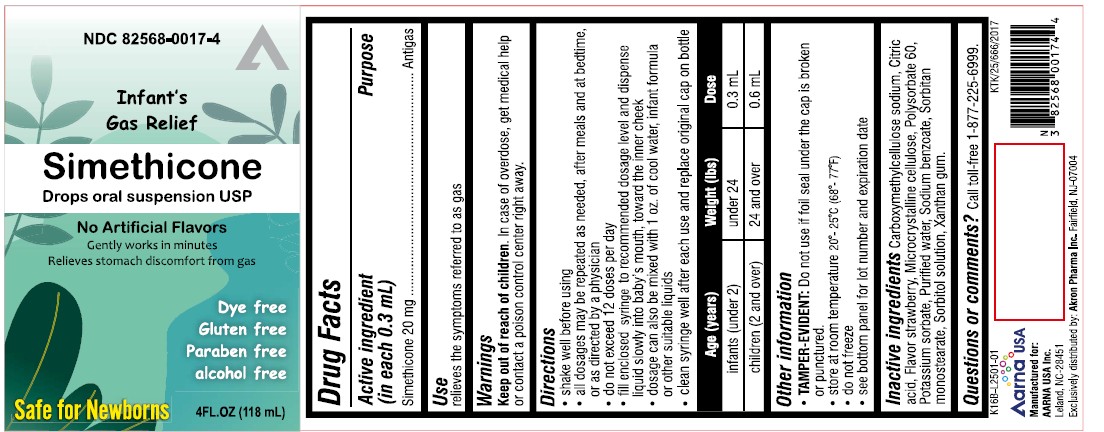

INDICATIONS & USAGE SECTION
Uses
relieves the symptoms referred to as gas
WARNINGS SECTION
Warnings
Keep out of reach of children.
In case of overdose get medical help or contact a Poison Control Center right away.
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each 0.3 mL)
Simethicone 20 mg
OTC - PURPOSE SECTION
Purpose
Antigas
DOSAGE & ADMINISTRATION SECTION
Directions
- shake well before using
- all dosages may be repeated as needed, alter meals and at bedtime, or as directed by a physician
- do not exceed 12 doses per day
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby's mouth, toward the inner cheek
- dosage can also be mixed with 1 oz. of cool water, infant formula or other suitable liquids
|
Age (years) |
Weight (lbs) |
Dose |
|
infants (under 2) |
under 24 |
0.3 mL |
|
children (2 and over) |
24 and over |
0.6 mL |
STORAGE AND HANDLING SECTION
Other information
TAMPER-EVIDENT*:**Do not use if printed seal under cap is broken or punctured
- store at room temperature
- do not freeze
- see bottom panel for lot number and expiration date
INACTIVE INGREDIENT SECTION
Inactive ingredients
Carboxymethylcellulose sodium, Citric acid, Flavor strawberry, Microcrystalline cellulose, Polysorbate 60, Potassium sorbate, Purified water, Sodium benzoate, Sorbitan monostearate, Sorbitol solution, Xanthan gum.
Questions or comments ?
Call toll-free 1-877-225-6999.
Manufactured for:
AARNA USA Inc.
Leland, NC-28451
