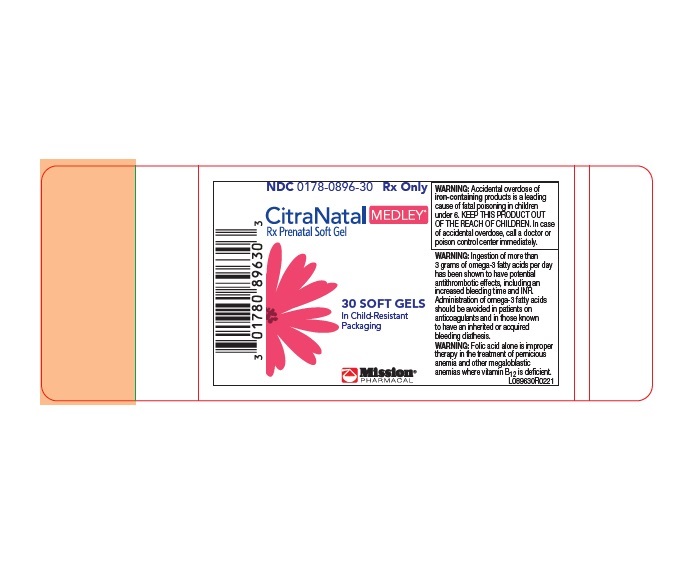
CitraNatal Medley
CitraNatal Medley
Approved
Approval ID
67c74737-6c8b-9aeb-e053-2a91aa0adf09
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Aug 13, 2025
Manufacturers
FDA
Mission Pharmacal Company
DUNS: 008117095
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Calcium citrate, Iron pentacarbonyl, Ferrous Fumarate, Cholecalciferol, .Alpha.-Tocopherol, Dl-, Pyridoxine hydrochloride, Folic Acid, and Doconexent
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code0178-0896
Product Classification
G
Generic Name
Calcium citrate, Iron pentacarbonyl, Ferrous Fumarate, Cholecalciferol, .Alpha.-Tocopherol, Dl-, Pyridoxine hydrochloride, Folic Acid, and Doconexent
Product Specifications
Route of AdministrationORAL
Effective DateAugust 13, 2025
FDA Product Classification
INGREDIENTS (16)
GLYCERINInactive
Code: PDC6A3C0OX
Classification: IACT
GELATINInactive
Code: 2G86QN327L
Classification: IACT
LECITHIN, SUNFLOWERInactive
Code: 834K0WOS5G
Classification: IACT
TITANIUM DIOXIDEInactive
Code: 15FIX9V2JP
Classification: IACT
BUTYLATED HYDROXYTOLUENEInactive
Code: 1P9D0Z171K
Classification: IACT
FD&C BLUE NO. 1Inactive
Code: H3R47K3TBD
Classification: IACT
ETHYL VANILLINInactive
Code: YC9ST449YJ
Classification: IACT
D&C RED NO. 33Inactive
Code: 9DBA0SBB0L
Classification: IACT
FERROUS FUMARATEActive
Quantity: 2 mg in 1 1
Code: R5L488RY0Q
Classification: ACTIM
CALCIUM CITRATEActive
Quantity: 62 mg in 1 1
Code: MLM29U2X85
Classification: ACTIR
IRONActive
Quantity: 27 mg in 1 1
Code: E1UOL152H7
Classification: ACTIB
CHOLECALCIFEROLActive
Quantity: 200 [iU] in 1 1
Code: 1C6V77QF41
Classification: ACTIB
DOCONEXENTActive
Quantity: 200 mg in 1 1
Code: ZAD9OKH9JC
Classification: ACTIB
PYRIDOXINEActive
Quantity: 12.5 mg in 1 1
Code: KV2JZ1BI6Z
Classification: ACTIB
.ALPHA.-TOCOPHEROLActive
Quantity: 15 [iU] in 1 1
Code: H4N855PNZ1
Classification: ACTIB
FOLIC ACIDActive
Quantity: 1 mg in 1 1
Code: 935E97BOY8
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 3/1/2018