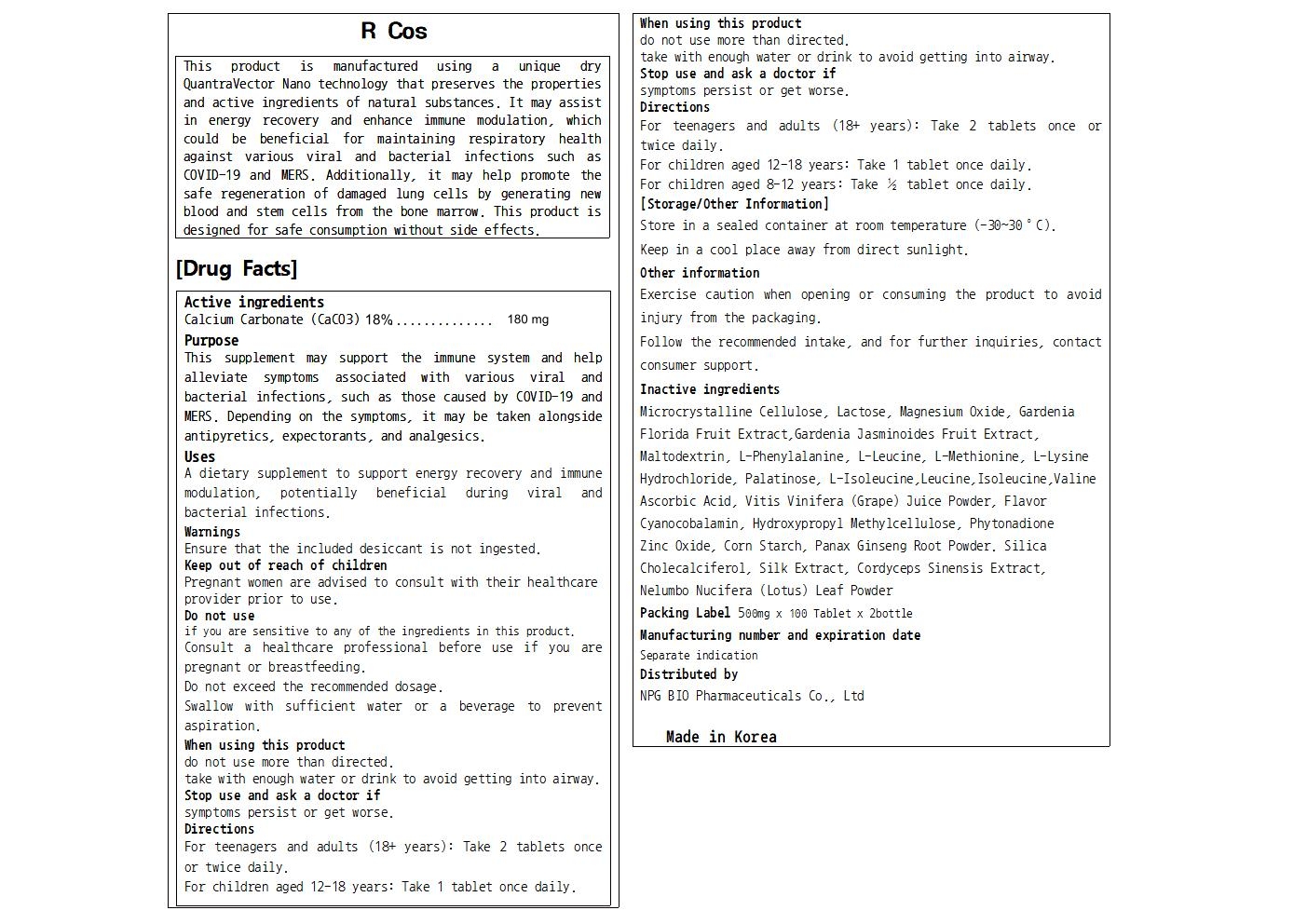
R Cos
84540-523 R Cos capsule
21469f58-1d70-774f-e063-6294a90af039
HUMAN OTC DRUG LABEL
Aug 27, 2025
NPG Bio Pharmaceuticals Co.,Ltd.
DUNS: 963309698
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Calcium Carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (26)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Label
 84540-501 R Cos
84540-501 R Cos
INDICATIONS & USAGE SECTION
Uses
A dietary supplement to support the immune system and help alleviate symptoms
associated with various viral and bacterial infections, such as those caused
by COVID-19 and MERS.
Depending on the stmptoms, it may be taken alongside antipyretics,
expectorants, and analgesics.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients
Calcium Carbonate (CaCO3) 18%...............180 mg
OTC - PURPOSE SECTION
Purposes
This supplement may support the immune system and help alleviate symptoms associated with various viral and bacterial infections, such as those caused by COVID-19 and MERS. Depending on the symptoms, it may be taken alongside antipyretics, expectorants, and analgesics.
OTC - DO NOT USE SECTION
Warnings
Do not use if you are sensitive to any of the ingredients in this product.
Consult a healthcare professional before use if you are pregnant or
breastfeeding.
Do not exceed the recommended dosage.
Swallow with sufficient water or a beverage to prevent aspiration.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Warnings
Keep out of reach of children
Pregnant women are advised to consult with their healthcare provider prior to
use.
OTC - STOP USE SECTION
Warnings
Stop use and ask a doctor if symptoms persist or get worse.
OTC - WHEN USING SECTION
Warnings
When using this product do not use more than directed.
take with enough water or drink to avoid getting into airway.
WARNINGS SECTION
Warnings
Ensure that the included desiccant is not ingested.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Microcrystalline Cellulose, Lactose, Magnesium Oxide, Gardenia Florida Fruit Extract,Gardenia Jasminoides Fruit Extract, Maltodextrin, L-Phenylalanine, L-Leucine, L-Methionine, L-Lysine Hydrochloride, Palatinose, L-Isoleucine,Leucine,Isoleucine,Valine
Ascorbic Acid, Vitis Vinifera (Grape) Juice Powder, Flavor
Cyanocobalamin, Hydroxypropyl Methylcellulose, Phytonadione
Zinc Oxide, Corn Starch, Panax Ginseng Root Powder. Silica
Cholecalciferol, Silk Extract, Cordyceps Sinensis Extract, Nelumbo Nucifera (Lotus) Leaf Powder
DOSAGE & ADMINISTRATION SECTION
Directions
For teenagers and adults (18+ years): Take 2 tablets once or twice daily.
For children aged 12-18 years: Take 1 tablet once daily.
For children aged 8-12 years: Take ½ tablet once daily.
