Hexatrione
Hexatrione
Approved
Approval ID
bc07ae9f-8a74-6bc8-e053-2995a90afc0d
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Jan 10, 2023
Manufacturers
FDA
Medexus Pharma, Inc.
DUNS: 078811131
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
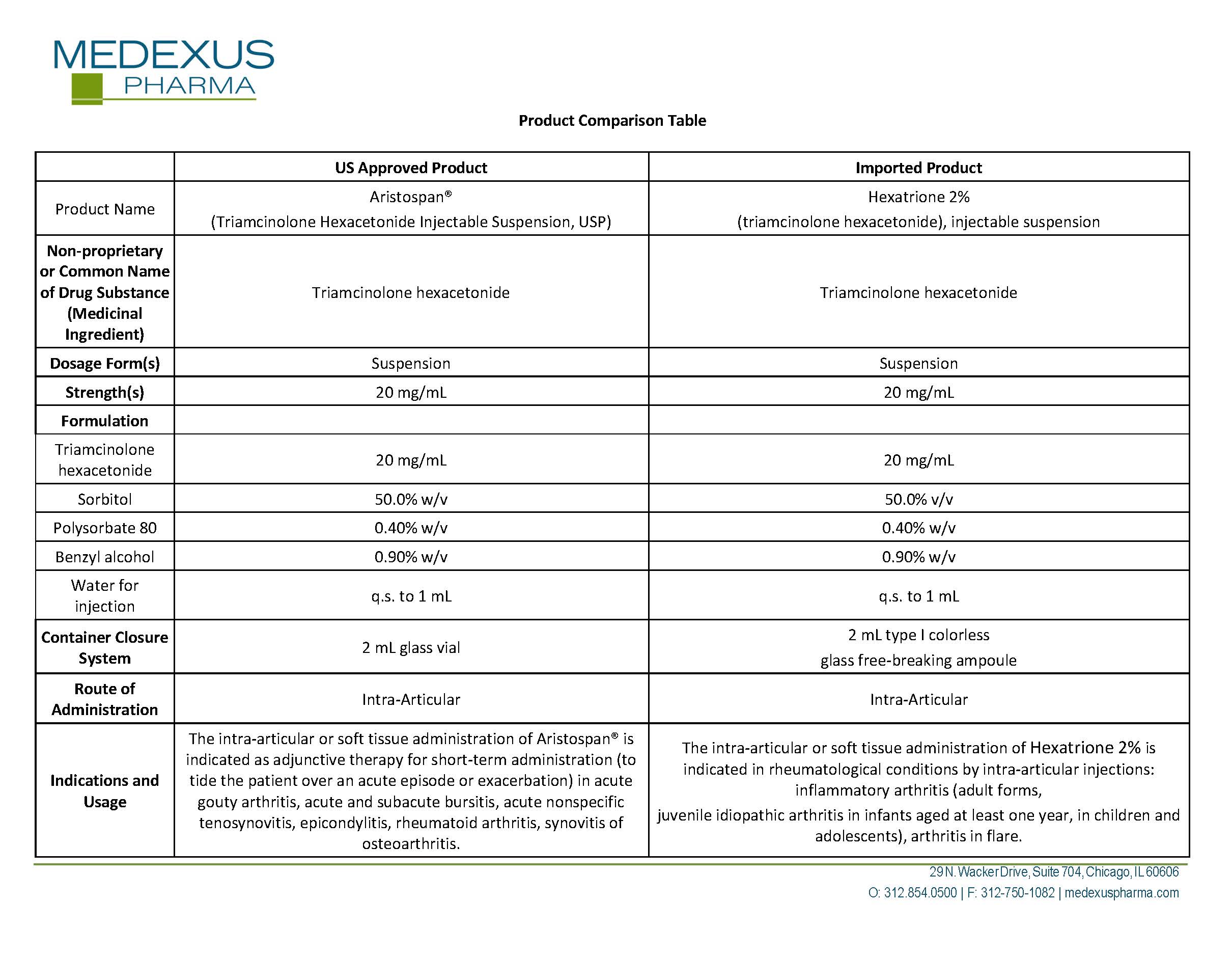
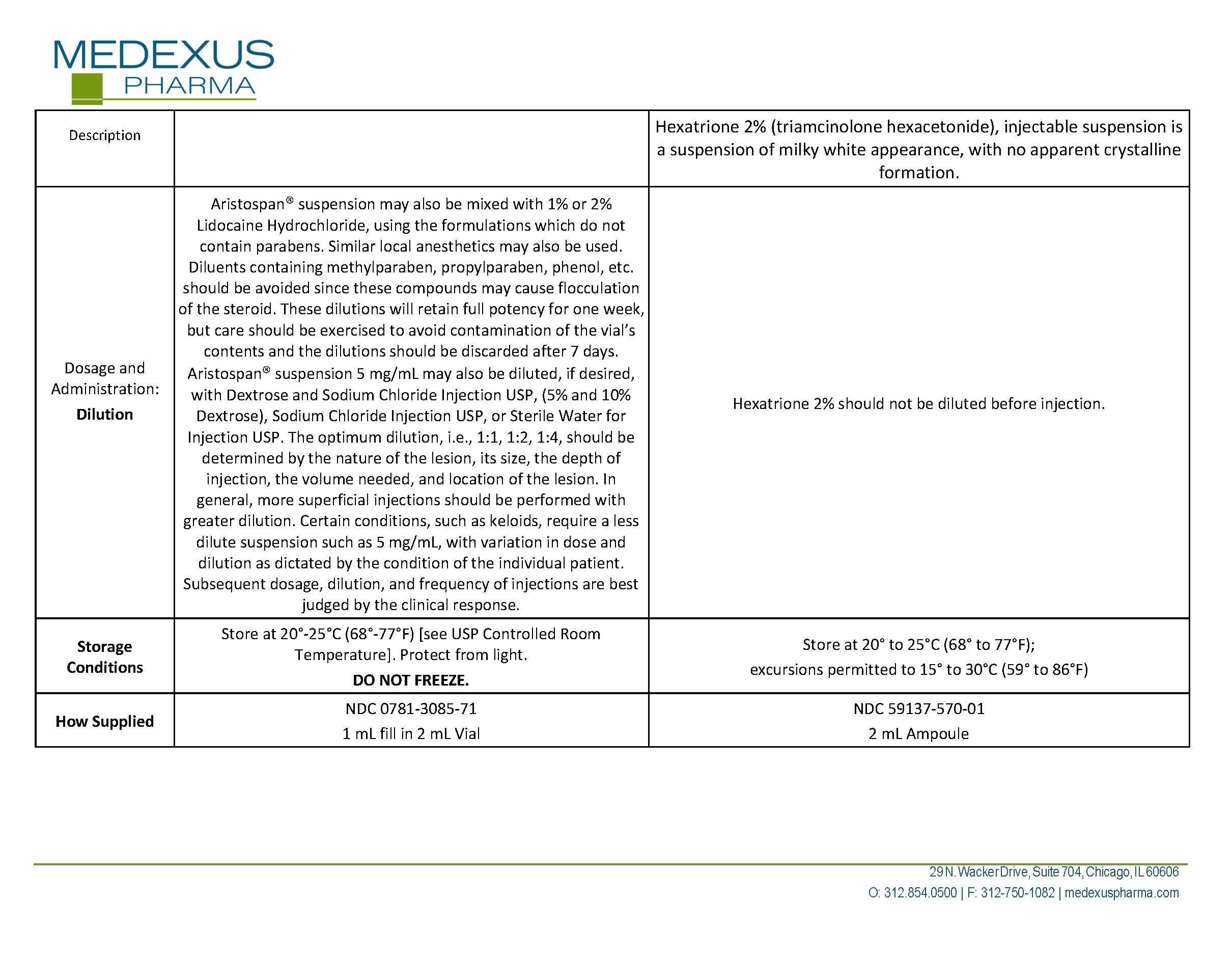
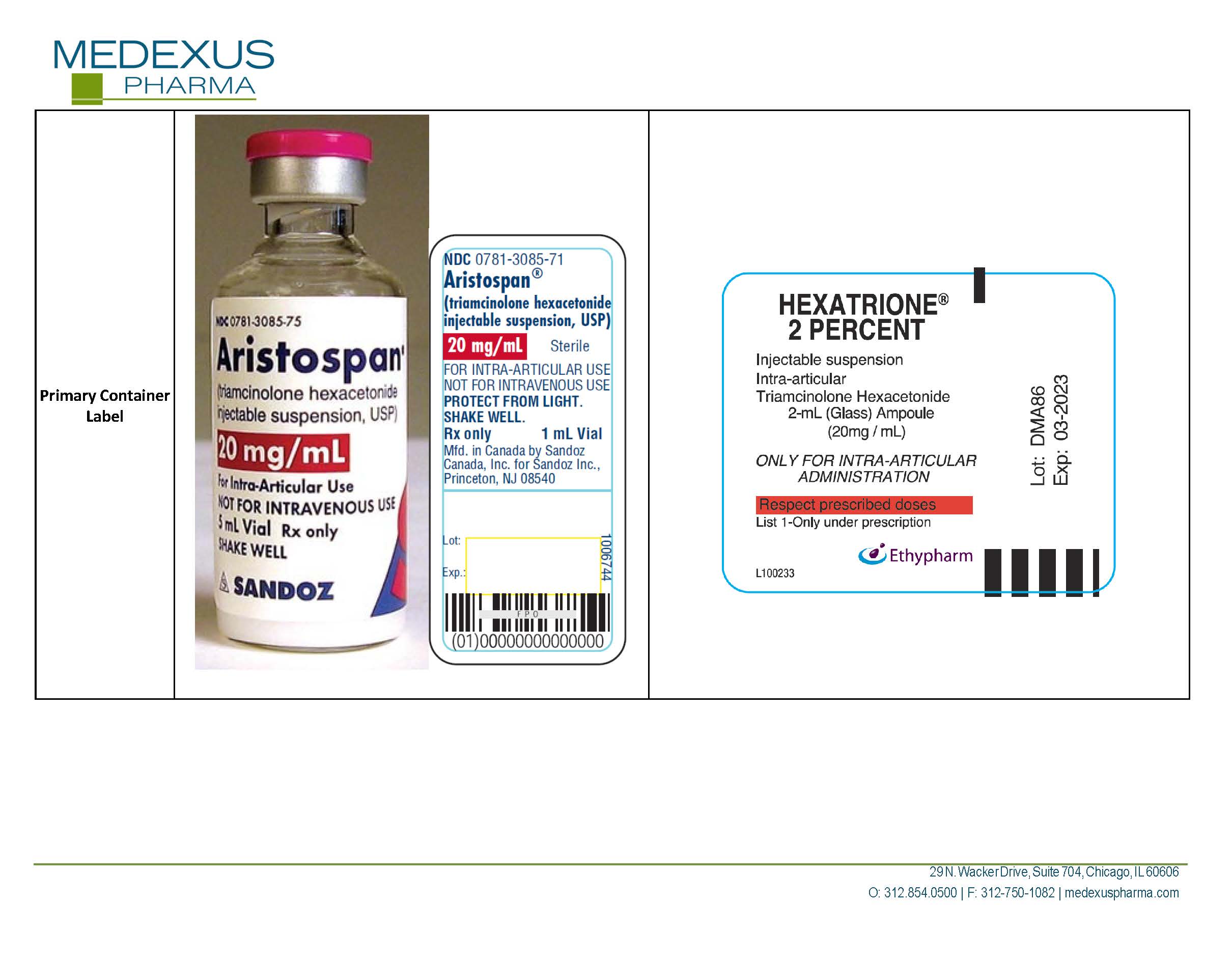
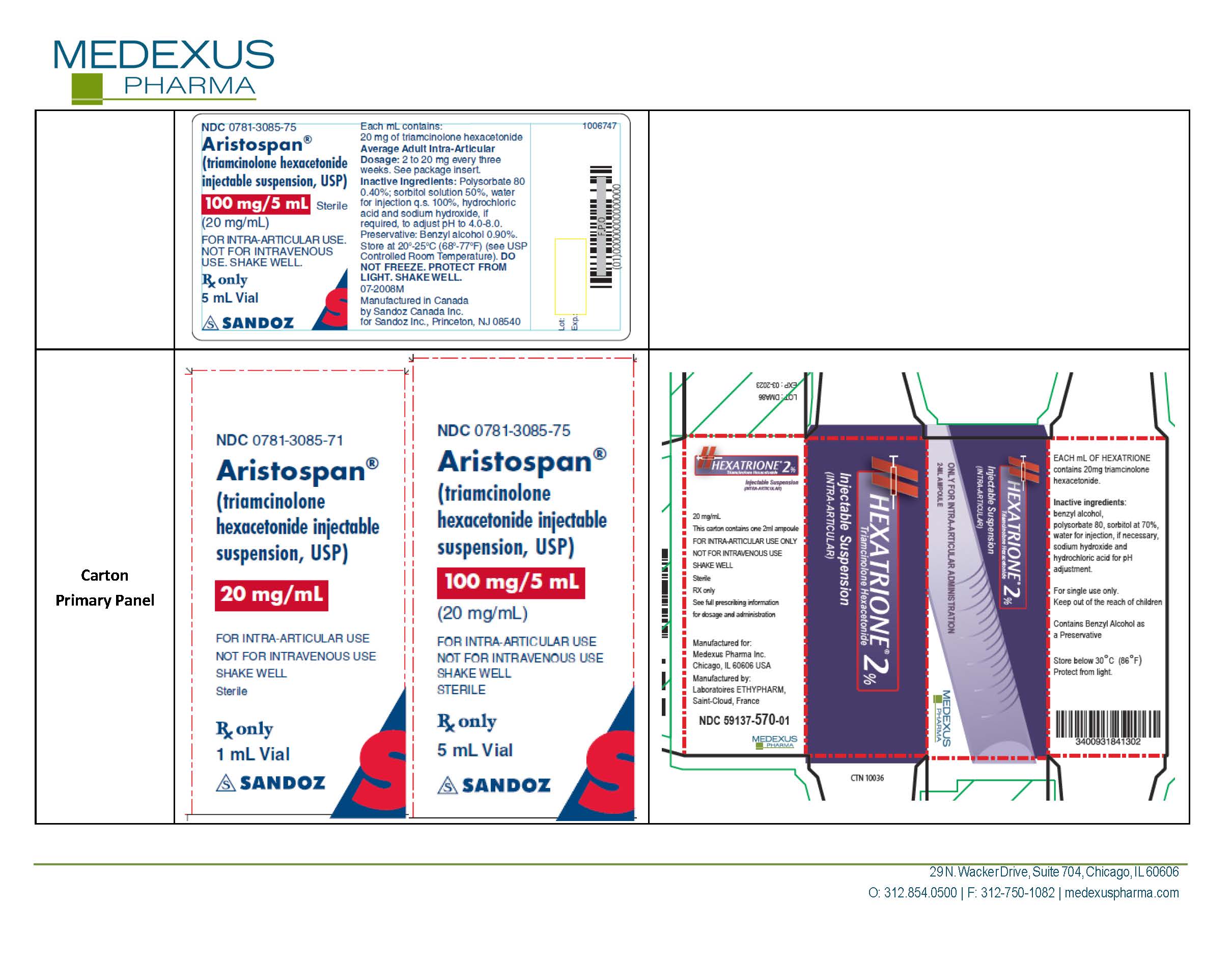
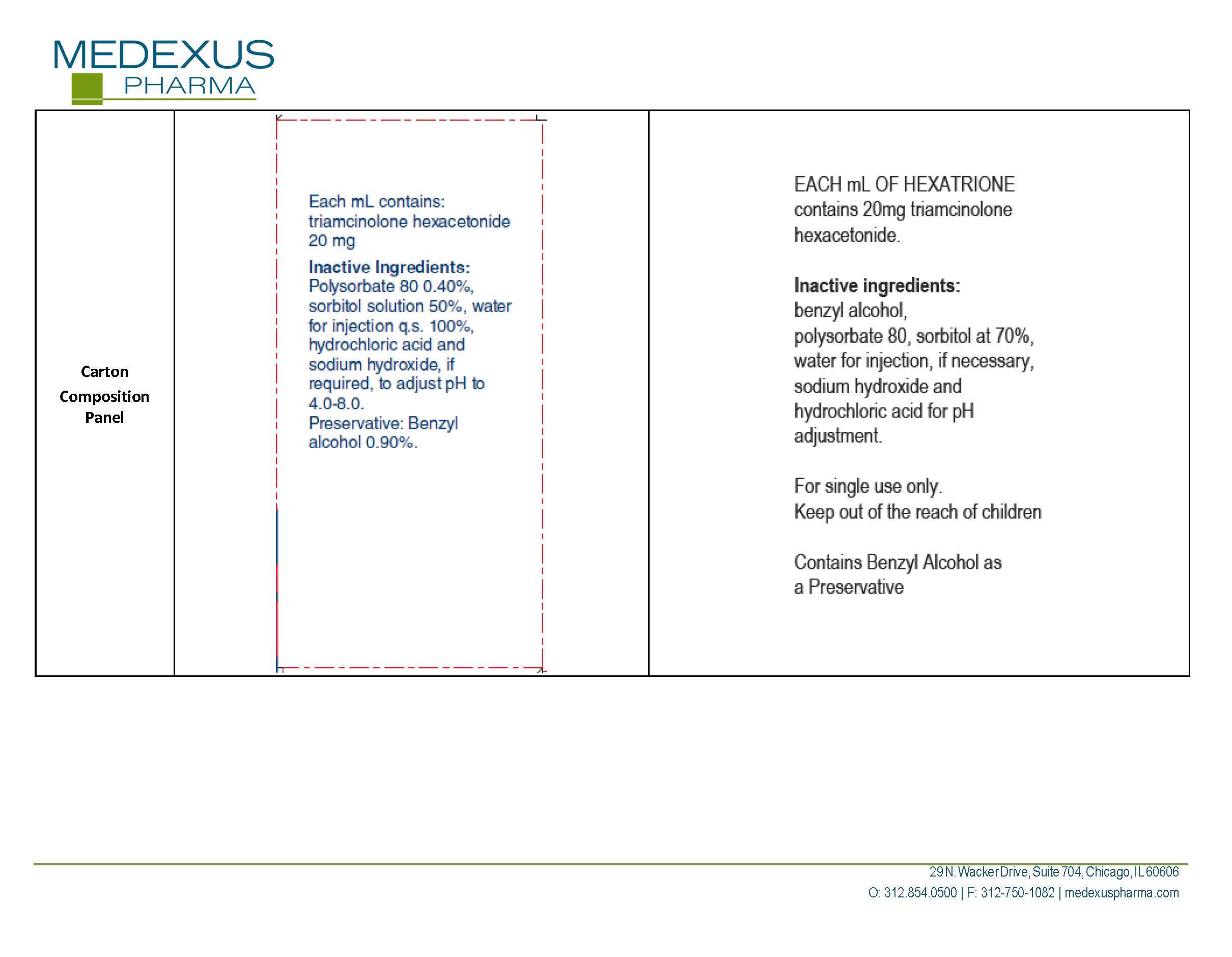
triamcinolone hexacetonide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code59137-570
Product Classification
G
Generic Name
triamcinolone hexacetonide
Product Specifications
Route of AdministrationINTRA-ARTICULAR
Effective DateJanuary 10, 2023
FDA Product Classification
INGREDIENTS (7)
SODIUM HYDROXIDEInactive
Code: 55X04QC32I
Classification: IACT
WATERInactive
Code: 059QF0KO0R
Classification: IACT
BENZYL ALCOHOLInactive
Code: LKG8494WBH
Classification: IACT
SORBITOL SOLUTION 70%Inactive
Code: 8KW3E207O2
Classification: IACT
HYDROCHLORIC ACIDInactive
Code: QTT17582CB
Classification: IACT
TRIAMCINOLONE HEXACETONIDEActive
Quantity: 40 mg in 2 mL
Code: I7GT1U99Y9
Classification: ACTIB
POLYSORBATE 80Inactive
Code: 6OZP39ZG8H
Classification: IACT
Drug Labeling Information
HEALTH CARE PROVIDER LETTER SECTION
LOINC: 71744-7Updated: 12/8/2021