AUVON Pain Relief Cream
83391-009
358c2632-3b90-1c54-e063-6294a90ad461
HUMAN OTC DRUG LABEL
May 22, 2025
SHENZHEN YUWEN E-COMMERCE CO., LTD.
DUNS: 544559614
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lidocaine
PRODUCT DETAILS
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

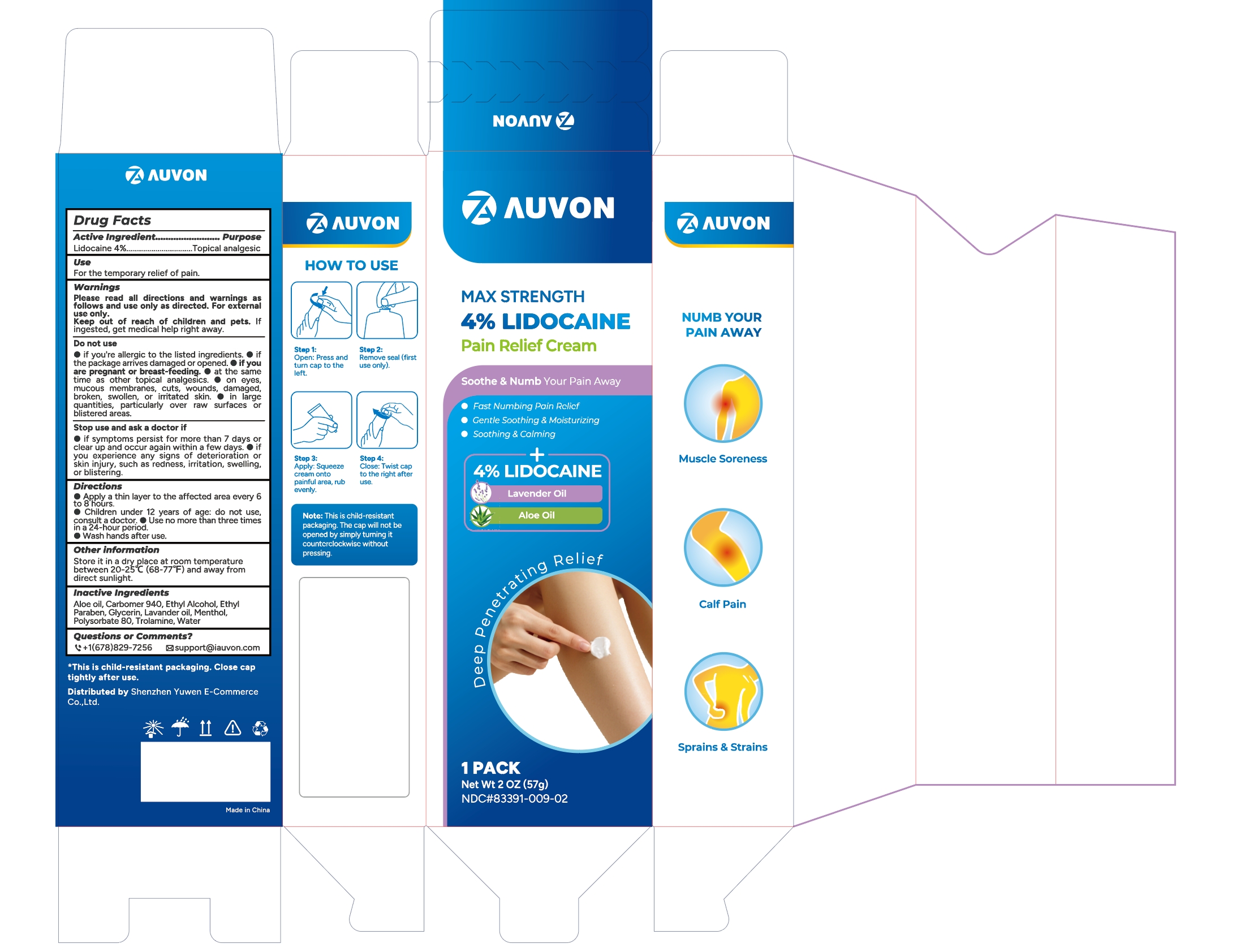

INDICATIONS & USAGE SECTION
For the temporay relief of pain
OTC - ACTIVE INGREDIENT SECTION
Lidocaine 4%
OTC - PURPOSE SECTION
Topical Analgestic
WARNINGS SECTION
Please read all directions and warnings as follows and use only as directed.
For external use only.
Keep out of reach of children and pets. If ingested, get medical help right
away.
SPL UNCLASSIFIED SECTION
Drug Facts
OTC - DO NOT USE SECTION
Do not use
if you're allergic to the listed ingredients.
if the package arrives damaged or opened.
if you are pregnant or breast-feeding.
at the same time as other topical analgesics.
on eyes, mucous membranes, cuts, wounds damaged, broken, swolen. or irritated skin.
In large quantities. particularly over raw surfaces or blistered area
OTC - STOP USE SECTION
Stop use and ask a doctor
if symptoms persist for more than / days or clear up and occur again within a
few davs.
if you experience any signs of deterioration or skin iniurv. such as redness, irritation, sweling, or bistering.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children and pets. If ingested, get medical help right away.
DOSAGE & ADMINISTRATION SECTION
Apply a thin layer to the affected area every 6 to 8 hours.
Children under 12 years of age:do not use, consult a doctor.
Use no more than three times in a day.
Wash hands after use.
STORAGE AND HANDLING SECTION
Store it in a dry place at room temperature between 20-25°C (68-77°F) and away from direct sunlight.
INACTIVE INGREDIENT SECTION
Aloe oil, Carbomer 940, Ethyl Alcohol, Ethyl Paraben, Glycerin, Lavander oil,
Menthol, Polysorbate 80, Trolamine, Water
