Tylenol Precise Pain Relieving Lidocaine 4%
Tylenol Precise Pain Relieving Patch Lidocaine 4%
36a95afd-97e5-2271-e063-6394a90aea18
HUMAN OTC DRUG LABEL
Sep 30, 2025
Kenvue Brands LLC
DUNS: 118772437
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lidocaine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
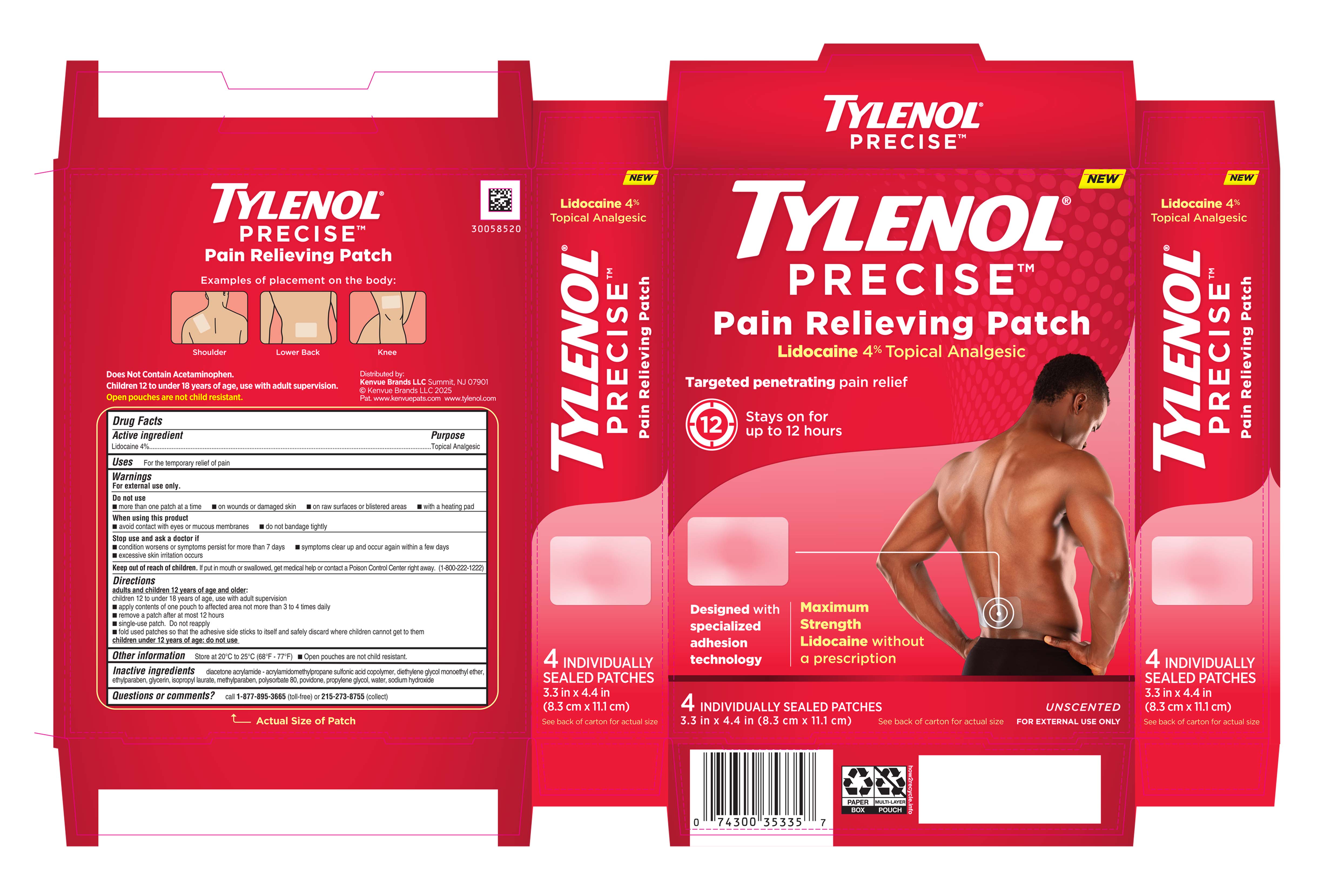
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - Tylenol Patch Label
NEW
TYLENOL**®**
PRECISE****TM
Pain Relieving Patch
Lidocaine 4% Topical Analgesic
Targeted penetrating pain relief
12
Stays on for
up to 12 hours
Designed with
specialized
adhesion
technology
Maximum
Strength
Lidocaine without
a prescription
4 INDIVIDUALLY SEALED PATCHES
3.3 in X 4.4 in (8.3 cm X 11.1 cm)
See back of carton for actual size
UNSCENTED
FOR EXTERNAL USE ONLY
INDICATIONS & USAGE SECTION
Uses
For the temporary relief of pain
SPL UNCLASSIFIED SECTION
Distributed by:
Kenvue Brands LLC
Summit, NJ 07901
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Lidocaine 4%
Purpose
Topical Analgesic
WARNINGS SECTION
Warnings
For external use only.
Do not use
- more than one patch at a time
- on wounds or damaged skin
- on raw surfaces or blistered areas
- with a heating pad
When using this product
- avoid contact with eyes or mucous membranes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive skin irritation occurs
Keep out of reach of children. If put in mouth or swallowed, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
DOSAGE & ADMINISTRATION SECTION
Directions
adults and children 12 years of age and older:
children 12 to under 18 years of age, use with adult supervision
- apply contents of one pouch to affected area not more than 3 to 4 times daily
- remove a patch after at most 12 hours
- single-use patch. Do not reapply
- fold used patches so that the adhesive side sticks to itself and safely discard where children cannot get to them
children under 12 years of age: do not use
STORAGE AND HANDLING SECTION
Other Information
Store at 20°C to 25°C (68°F - 77°F) ■ Open pouches are not child resistant.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
diacetone acrylamide - acrylamidomethylpropane sulfonic acid copolymer, diethylene glycol monoethyl ether, ethylparaben, glycerin, isopropyl laurate, methylparaben, polysorbate 80, povidone, propylene glycol, water, sodium hydroxide
OTC - QUESTIONS SECTION
Questions or comments?
call1-877-895-3665(toll-free) or215-273-8755 (collect)
