Clobazam
These highlights do not include all the information needed to use CLOBAZAM TABLETS safely and effectively. See full prescribing information for CLOBAZAM TABLETS. CLOBAZAM tablets, for oral use, CIV Initial U.S. Approval: 2011
721dde68-db83-4dad-8649-8046cb455372
HUMAN PRESCRIPTION DRUG LABEL
Mar 20, 2024
Micro Labs Limited
DUNS: 862174955
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Clobazam
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Clobazam
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 42571-315-01
Clobazam Tablets CIV
10 mg
DISPENSE THE ENCLOSED MEDICATION GUIDE
WITH EACH PRESCRIPTION
Rx Only
100 Tablets
Micro Labs Limited

NDC 42571-316-01
Clobazam Tablets CIV
20 mg
DISPENSE THE ENCLOSED MEDICATION GUIDE
WITH EACH PRESCRIPTION
Rx Only
100 Tablets
Micro Labs Limited

BOXED WARNING SECTION
**WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND
ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS****See full prescribing information for complete boxed warning.**
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Clobazam tablet is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older.
Clobazam tablet is a benzodiazepine indicated for adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older ( 1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Clobazam tablets are contraindicated in patients with a history of hypersensitivity to the drug or its ingredients. Hypersensitivity reactions have included serious dermatological reactions [see Warnings and Precautions (5.6, 5.7)].
History of hypersensitivity to the drug or its ingredients ( 4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including clobazam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe clobazam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise both patients and caregivers about the risks of respiratory depression and sedation when clobazam is used with opioids [seeDrug Interactions (7.1)].
5.2 Abuse, Misuse, and Addiction
The use of benzodiazepines, including clobazam, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death [see Drug Abuse and Dependence ( 9.2)].
Before prescribing clobazam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of clobazam, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of clobazam along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
5.3 Dependence and Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clobazam or reduce the dosage [see Dosage and Administration (2.2)]. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including clobazam, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of clobazam after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures ) [see Drug Abuse and Dependence (9.3)].
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months [see Drug Abuse and Dependence (9.3)].
5.4 Potentiation of Sedation from Concomitant Use with Central Nervous
System Depressants
Since clobazam has a central nervous system (CNS) depressant effect, patients or their caregivers should be cautioned against simultaneous use with other CNS depressant drugs or alcohol, and cautioned that the effects of other CNS depressant drugs or alcohol may be potentiated [seeDrug Interactions (7.2)] .
5.5 Somnolence or Sedation
Clobazam causes somnolence and sedation. In clinical trials, somnolence or sedation was reported at all effective doses and was dose-related.
In general, somnolence and sedation begin within the first month of treatment and may diminish with continued treatment. Prescribers should monitor patients for somnolence and sedation, particularly with concomitant use of other central nervous system depressants. Prescribers should caution patients against engaging in hazardous activities requiring mental alertness, such as operating dangerous machinery or motor vehicles, until the effect of clobazam is known.
5.6 Serious Dermatological Reactions
Serious skin reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported with clobazam in both children and adults during the postmarketing period. Patients should be closely monitored for signs or symptoms of SJS/TEN, especially during the first 8 weeks of treatment initiation or when re-introducing therapy. Clobazam should be discontinued at the first sign of rash, unless the rash is clearly not drug-related. If signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered [seeContraindications (4)].
5.7 Drug Reaction with Eosinophilia and Systemic Symptoms
(DRESS)/Multiorgan Hypersensitivity
Drug Reaction with Eosinophilia andSystemicSymptoms (DRESS), also known as multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including clobazam.Theseevents can be fatal or life- threatening, particularly if diagnosis and treatment do not occur as early as possible. DRESS typically, although notexclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis,nephritis, hematological abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. Because thisdisorderis variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations ofhypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patientshouldbe evaluated immediately. Clobazam should be discontinued if an alternative etiology for the signs or symptoms cannot be established [see Contraindications (4)].
5.8 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including clobazam, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted relative risk 1.8, 95% confidence interval [CI]: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed. Table 2 shows absolute and relative risk by indication for all evaluated AEDs.
Table 2. Risk by Indication for Antiepileptic Drugs in the Pooled Analysis
|
Indication |
Placebo Patients with Events per 1000 Patients |
Drug Patients with Events per 1000 Patients |
Relative Risk: Incidence of Drug Events in Drug Patients/Incidence in Placebo Patients |
Risk Difference: Additional Drug Patients with Events per 1000 Patients |
|
Epilepsy |
1 |
3.4 |
3.5 |
2.4 |
|
Psychiatric |
5.7 |
8.5 |
1.5 |
2.9 |
|
Other |
1 |
1.8 |
1.9 |
0.9 |
|
Total |
2.4 |
4.3 |
1.8 |
1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing clobazam or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
5.9 Neonatal Sedation and Withdrawal Syndrome
Use of clobazam late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate [ see Use in Specific Populations (8.1)]. Monitor neonates exposed to clobazam during pregnancy or labor for signs of sedation and monitor neonates exposed to clobazam during pregnancy for signs of withdrawal; manage these neonates accordingly.
-
Somnolence or Sedation: Monitor for central nervous system (CNS) depression. Risk may be increased with concomitant use of other CNS depressants ( 5.4, 5.5)
-
Serious Dermatological Reactions (including Stevens-Johnson syndrome and toxic epidermal necrolysis): Discontinue clobazam tablet at first sign of rash unless the rash is clearly not drug-related ( 5.6)
-
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity: Discontinue if no alternative etiology ( 5.7)
-
Suicidal Behavior and Ideation: Monitor for suicidal thoughts or behaviors ( 5.8)
-
Neonatal Sedation and Withdrawal Syndrome: Clobazam tablet use during pregnancy can result in neonatal sedation and/or neonatal withdrawal ( 5.9, 8.1)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
Clinically significant adverse reactions that appear in other sections of the labeling include the following:
• Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
• Abuse, Misuse, and Addiction [see Warnings and Precautions (5.2)]
• Dependence and Withdrawal Reactions [see Warnings and Precautions (5.3)]
• Potentiation of Sedation from Concomitant Use with Central Nervous System
Depressants [see Warnings and Precautions (5.4)]
• Somnolence or Sedation [see Warnings and Precautions (5.5)]
• Serious Dermatological Reactions [see Contraindications (4), Warnings and Precautions (5.6)]
• Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan
Hypersensitivity [see Warnings and Precautions (5.7)]
• Suicidal Behavior and Ideation [see Warnings and Precautions (5.8)]
• Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During its development for the adjunctive treatment of seizures associated with LGS, clobazam was administered to 333 healthy volunteers and 300 patients with a current or prior diagnosis of LGS, including 197 patients treated for 12 months or more. The conditions and duration of exposure varied greatly and included single- and multiple- dose clinical pharmacology studies in healthy volunteers and two double-blind studies in patients with LGS (Study 1 and 2) [seeClinical Studies (14)] . Only Study 1 included a placebo group, allowing comparison of adverse reaction rates on clobazam at several doses to placebo.
Adverse Reactions Leading to Discontinuation in an LGS Placebo Controlled Clinical Trial (Study 1)
The adverse reactions associated with clobazam treatment discontinuation in ≥1% of patients in decreasing order of frequency included lethargy, somnolence, ataxia, aggression, fatigue, and insomnia.
Most Common Adverse Reactions in an LGS Placebo Controlled Clinical Trial (Study 1)
Table 3 lists the adverse reactions that occurred in ≥5% of clobazam-treated patients (at any dose), and at a rate greater than placebo-treated patients, in the randomized, double-blind, placebo-controlled, parallel group clinical study of adjunctive AED therapy for 15 weeks (Study 1).
Table 3. Adverse Reactions Reported for ≥5% of Patients and More Frequently than Placebo in Any Treatment Group
|
Placebo |
Clobazam Dose Level |
All Clobazam N=179% | |||
|
Low****a |
MediumbN=62% |
High****c | |||
|
Gastrointestinal Disorders | |||||
|
Vomiting |
5 |
9 |
5 |
7 |
7 |
|
Constipation |
0 |
2 |
2 |
10 |
5 |
|
Dysphagia |
0 |
0 |
0 |
5 |
2 |
|
General Disorders and Administration Site Conditions | |||||
|
Pyrexia |
3 |
17 |
10 |
12 |
13 |
|
Irritability |
5 |
3 |
11 |
5 |
7 |
|
Fatigue |
2 |
5 |
5 |
3 |
5 |
|
Infections and Infestations | |||||
|
Upper respiratory tract infection |
10 |
10 |
13 |
14 |
12 |
|
Pneumonia |
2 |
3 |
3 |
7 |
4 |
|
Urinary tract infection |
0 |
2 |
5 |
5 |
4 |
|
Bronchitis |
0 |
2 |
0 |
5 |
2 |
|
Metabolism and Nutrition Disorders | |||||
|
Decreased appetite |
3 |
3 |
0 |
7 |
3 |
|
Increased appetite |
0 |
2 |
3 |
5 |
3 |
|
Nervous System Disorders | |||||
|
Somnolence or Sedation |
15 |
17 |
27 |
32 |
26 |
|
Somnolence |
12 |
16 |
24 |
25 |
22 |
|
Sedation |
3 |
2 |
3 |
9 |
5 |
|
Lethargy |
5 |
10 |
5 |
15 |
10 |
|
Drooling |
3 |
0 |
13 |
14 |
9 |
|
Ataxia |
3 |
3 |
2 |
10 |
5 |
|
Psychomotor hyperactivity |
3 |
3 |
3 |
5 |
4 |
|
Dysarthria |
0 |
2 |
2 |
5 |
3 |
|
Psychiatric Disorders | |||||
|
Aggression |
5 |
3 |
8 |
14 |
8 |
|
Insomnia |
2 |
2 |
5 |
7 |
5 |
|
Respiratory Disorders | |||||
|
Cough |
0 |
3 |
5 |
7 |
5 |
aMaximum daily dose of 5 mg for ≤30 kg body weight; 10 mg for >30 kg body weight
bMaximum daily dose of 10 mg for ≤30 kg body weight; 20 mg for >30 kg body weight
cMaximum daily dose of 20 mg for ≤30 kg body weight; 40 mg for >30 kg body weight
6.2 Postmarketing Experience
These reactions are reported voluntarily from a population of uncertain size; therefore, it is not possible to estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions are categorized by system organ class.
Blood Disorders:Anemia, eosinophilia, leukopenia, thrombocytopenia
Eye Disorders:Diplopia, vision blurred
Gastrointestinal Disorders:Abdominal distention
General Disorders and Administration Site Conditions:Hypothermia
Investigations:Hepatic enzyme increased
Musculoskeletal:Muscle spasms
Psychiatric Disorders:Agitation, anxiety, apathy, confusional state, depression, delirium, delusion, hallucination
Renal and Urinary Disorders:Urinary retention
Respiratory Disorders:Aspiration, respiratory depression
Skin and Subcutaneous Tissue Disorders:Rash, urticaria, angioedema, and facial and lip edema
Adverse reactions that occurred at least 10% more frequently than placebo in any clobazam tablet dose included constipation, somnolence or sedation, pyrexia, lethargy, and drooling ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Micro Labs USA, Inc. at 1-855-839-8195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Opioids
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and follow patients closely for respiratory depression and sedation [seeWarnings and Precautions (5.1)] .
7.2 CNS Depressants and Alcohol
Concomitant use of clobazam with other CNS depressants may increase the risk of sedation and somnolence [seeWarnings and Precautions (5.4)] .
Alcohol, as a CNS depressant, will interact with clobazam in a similar way and also increases clobazam’s maximum plasma exposure by approximately 50%. Therefore, caution patients or their caregivers against simultaneous use with other CNS depressant drugs or alcohol, and caution that the effects of other CNS depressant drugs or alcohol may be potentiated [see Warnings and Precautions (5.4)] .
7.3 Effect of Clobazam Tablet on Other Drugs
Hormonal Contraceptives
Clobazam is a weak CYP3A4 inducer. As some hormonal contraceptives are metabolized by CYP3A4, their effectiveness may be diminished when given with clobazam. Additional non-hormonal forms of contraception are recommended when using clobazam [seeClinical Pharmacology (12.3), Patient Counseling Information (17)] .
Drugs Metabolized by CYP2D6
Clobazam inhibits CYP2D6. Dose adjustment of drugs metabolized by CYP2D6 may be necessary [see Clinical Pharmacology (12.3)] .
7.4 Effect of Other Drugs on Clobazam Tablet
Strong and moderate inhibitors of CYP2C19
Strong and moderate inhibitors of CYP2C19 may result in increased exposure to N-desmethylclobazam, the active metabolite of clobazam. This may increase the risk of dose-related adverse reactions. Dosage adjustment of clobazam may be necessary when co-administered with strong CYP2C19 inhibitors (e.g., fluconazole, fluvoxamine, ticlopidine) or moderate CYP2C19 inhibitors (e.g., omeprazole) [see Clinical Pharmacology (12.3)] .
- Alcohol: Increases blood levels of clobazam by about 50% ( 7.2)
- Drugs metabolized by CYP2D6: Lower doses of these drugs may be required when used concomitantly with clobazam ( 7.3)
- Strong or Moderate CYP2C19 Inhibitors: Dosage adjustment of clobazam tablet may be necessary ( 7.4)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions ( 5.7) 3/2024
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
10 mg- White to off**-**white, oval shaped tablets with a functional score line on one face and "1" and "0" debossed on the other face.
20 mg- White to off**-**white, oval shaped tablets with a functional score line on one face and "2" and "0" debossed on the other face.
- Tablet: 10 mg and 20 mg with a functional score ( 3)
DESCRIPTION SECTION
11 DESCRIPTION
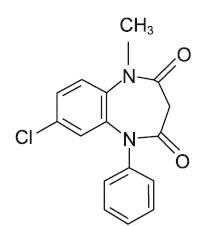
Clobazam tablet is a benzodiazepine derivative. The chemical name of clobazam is 7-Chloro-1-methyl-5-phenyl-1H-1,5 benzodiazepine-2,4(3H,5H)-dione with the following structure:
Clobazam is a white or almost white, crystalline powder with a slightly bitter
taste; is slightly soluble in water, sparingly soluble in ethanol, and freely
soluble in methylenechloride. The melting range of clobazam is from 182ºC to
185ºC. The molecular formula is C 16H 13O 2N 2Cl and the molecular weight is
300.7.
Each clobazam tablet contains 10 mg or 20 mg of clobazam. Tablets also contain as inactive ingredients: colloidal silicon dioxide, lactose monohydrate, lactose anhydrous, magnesium stearate, maize starch 5%, pregelatinized starch, and talc.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The effectiveness of clobazam for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome was established in two multicenter controlled studies (Study 1 and Study 2). Both studies were similar in terms of disease characteristics and concomitant AED treatments. The most common concomitant AED treatments at baseline included: valproate, lamotrigine, levetiracetam, and topiramate.
Study 1
Study 1 (N=238) was a randomized, double-blind, placebo-controlled study consisting of a 4-week baseline period followed by a 3-week titration period and 12-week maintenance period. Patients age 2 to 54 years with a current or prior diagnosis of LGS were stratified into 2 weight groups (12.5 kg to ≤30 kg or >30 kg) and then randomized to placebo or one of three target maintenance doses of clobazam according to Table 5.
Table 5. Study 1 Total Daily Dose
|
≤30 kg Body Weight |
>30 kg Body Weight | |
|
Low Dose |
5 mg daily |
10 mg daily |
|
Medium Dose |
10 mg daily |
20 mg daily |
|
High Dose |
20 mg daily |
40 mg daily |
Doses above 5 mg/day were administered in two divided doses.
The primary efficacy measure was the percent reduction in the weekly frequency of drop seizures (atonic, tonic, or myoclonic), also known as drop attacks, from the 4-week baseline period to 12-week maintenance period.
The pre-dosing baseline mean weekly drop seizure frequency was 98, 100, 61, and 105 for the placebo, low-, medium-, and high-dose groups, respectively. Figure 1 presents the mean percent reduction in weekly drop seizures from this baseline. All dose groups of clobazam were statistically superior (p≤0.05) to the placebo group. This effect appeared to be dose dependent.
Figure 1. Mean Percent Reduction from Baseline in Weekly Drop Seizure Frequency (Study 1)
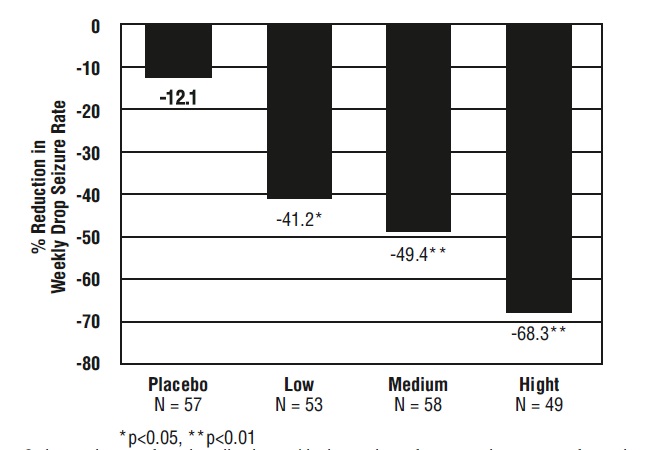
Figure 2 shows changes from baseline in weekly drop seizure frequency by category for patients treated with clobazam and placebo in Study 1. Patients in whom the seizure frequency increased are shown at left as “worse.” Patients in whom the seizure frequency decreased are shown in five categories.
Figure 2. Drop Seizure Response by Category for Clobazam and Placebo (Study 1)

There was no evidence that tolerance to the therapeutic effect of clobazam developed during the 3-month maintenance period.
Study 2
Study 2 (N=68) was a randomized, double-blind comparison study of high- and low-dose clobazam, consisting of a 4-week baseline period followed by a 3-week titration period and 4-week maintenance period. Patients age 2 to 25 years with a current or prior diagnosis of LGS were stratified by weight, then randomized to either a low or high dose of clobazam, and then entered a 3-week titration period.
The primary efficacy measure was the percent reduction in the weekly frequency of drop seizures (atonic, tonic, or myoclonic), also known as drop attacks, from the 4-week baseline period to the 4-week maintenance period.
A statistically significantly greater reduction in seizure frequency was observed in the high-dose group compared to the low-dose group (median percent reduction of 93% vs 29%; p<0.05).
SPL MEDGUIDE SECTION
MEDICATION GUIDE
|
Clobazam (KLOE-ba-zam) Tablets, CIV |
|
** What is the most important information I should know about clobazam tablets?** **• Clobazam tablets are a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death.**Get emergency help right away if any of the following happens: o shallow or slowed breathing • Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines, including clobazam tablets, which can lead to overdose and serious side effects including coma and death. oSerious side effects including coma and death have happened in people who
have abused or misused benzodiazepines, including clobazam tablets.These
serious side effects may also include delirium, paranoia, suicidal thoughts or
actions, seizures, and difficulty breathing.Call your healthcare provider
or go to the nearest hospital emergency room right away if you get any of
these serious side effects. •**Physical dependence and withdrawal reactions.Clobazam tablets can cause
physical dependence and withdrawal reactions. • Clobazam tablets can make you sleepy or dizzy and can slow your thinking
and motor skills. •**Serious skin reactions have been seen when clobazam tablets are taken with
other medicines and may require stopping its use.**Do not stop taking clobazam
tablets without first talking to your healthcare provider. o thoughts about suicide or dying o attempts to commit suicide Call your healthcare provider between visits as needed, especially if you are worried about symptoms. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus). Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. |
|
** What are clobazam tablets?** • Clobazam tablets are a prescription medicine used along with other medicines to treat seizures associated with Lennox-Gastaut syndrome in people 2 years of age or older. •**Clobazam tablet is a federally controlled substance (C-IV) because it contains clobazam that can be abused or lead to dependence.**Keep clobazam tablets in a safe place to prevent misuse and abuse. Selling or giving away clobazam tablets may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines, or street drugs. It is not known if clobazam tablets are safe and effective in children less than 2 years old. |
|
** Do not take clobazam tablet if you:** • are allergic to clobazam or any of the ingredients in clobazam tablets. See the end of this Medication Guide for a complete list of ingredients in clobazam tablets. |
|
** Before you take clobazam tablets, tell your healthcare provider about all
your medical conditions, including if you:** |
|
** How should I take clobazam tablets?** |
|
** What should I avoid while taking clobazam tablets?** See**“What is the most important information I should know about clobazam tablets?”** |
|
** What are the possible side effects of clobazam tablets?** Clobazam tablets may cause serious side effects, including: See “What is the most important information I should know about clobazam tablets?” ** The most common side effects of clobazam tablets include:** These are not all the possible side effects of clobazam tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
** How should I store clobazam tablets?** • Keep clobazam tablets in a dry place. |
|
** General information about the safe and effective use of clobazam tablets. ** Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use clobazam tablets for a condition for which it was not prescribed. Do not give clobazam tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about clobazam tablets that is written for health professionals. |
|
What are the ingredients in clobazam tablets? **Active ingredient:**clobazam For more information call1-855-839-8195. This Medication Guide has been approved by the U.S. Food and Drug Administration Manufactured by: Manufactured for: Rev. 03/2024 |
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
A daily dose of clobazam greater than 5 mg should be administered in divided doses twice daily; a 5 mg daily dose can be administered as a single dose. Dose patients according to body weight. Individualize dosing within each body weight group, based on clinical efficacy and tolerability. Each dose in Table 1 (e.g., 5 to 20 mg in ≤30 kg weight group) has been shown to be effective, although effectiveness increases with increasing dose [seeClinical Studies (14)]. Do not proceed with dose escalation more rapidly than weekly, because serum concentrations of clobazam and its active metabolite require 5 and 9 days, respectively, to reach steady-state.
Table 1. Recommended Total Daily Dosing by Weight Group
|
≤30 kg Body Weight |
>30 kg Body Weight | |
|
Starting Dose |
5 mg |
10 mg |
|
Starting Day 7 |
10 mg |
20 mg |
|
Starting Day 14 |
20 mg |
40 mg |
2.2 Discontinuation or Dosage Reduction of Clobazam Tablet
To reduce the risk of withdrawal reactions, increased seizure frequency, and status epilepticus, use a gradual taper to discontinue clobazam tablets or reduce the dosage. Taper by decreasing the total daily dose by 5 to 10 mg/day on a weekly basis until discontinued. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly [see Warnings and Precautions (5.3)and Drug Abuse and Dependence (9.3)].
2.3 Important Administration Instructions
Clobazam Tablet Oral Administration
Clobazam tablets can be taken with or without food.
Clobazam tablets can be administered whole, broken in half along the score, or crushed and mixed in applesauce.
2.4 Dosage Adjustments in Geriatric Patients
Plasma concentrations at any given dose are generally higher in the elderly: proceed slowly with dose escalation. The starting dose should be 5 mg/day for all elderly patients. Then titrate elderly patients according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, an additional titration to the maximum dose (20 mg/day or 40 mg/day, depending on weight) may be started on day 21 [see Use in Specific Populations (8.5)].
2.5 Dosage Adjustments in CYP2C19 Poor Metabolizers
In CYP2C19 poor metabolizers, levels of N-desmethylclobazam, clobazam’s active metabolite, will be increased. Therefore, in patients known to be CYP2C19 poor metabolizers, the starting dose should be 5 mg/day and dose titration should proceed slowly according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, an additional titration to the maximum dose (20 mg/day or 40 mg/day, depending on the weight group) may be started on day 21 [see Use in Specific Populations ( 8.6 ), Clinical Pharmacology ( 12.5 )] .
2.6 Patients with Renal Impairment
No dose adjustment is required for patients with mild and moderate renal impairment. There is no experience with clobazam in patients with severe renal impairment or end stage renal disease (ESRD). It is not known if clobazam or its active metabolite, N-desmethylclobazam, is dialyzable [seeUse in Specific Populations (8.7), Clinical Pharmacology (12.3)] .
2.7 Dosage Adjustments in Patients with Hepatic Impairment
Clobazam is hepatically metabolized; however, there are limited data to characterize the effect of hepatic impairment on the pharmacokinetics of clobazam. For this reason, proceed slowly with dosing escalations. For patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9), the starting dose should be 5 mg/day in both weight groups. Then titrate patients according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, start an additional titration on day 21 to the maximum dose (20 mg/day or 40 mg/day, depending on the weight group). There is inadequate information about metabolism of clobazam in patients with severe hepatic impairment. Therefore no dosing recommendation in those patients can be given [seeUse in Specific Populations (8.8), Clinical Pharmacology (12.3)] .
• For doses above 5 mg/day administer in two divided doses ( 2.1)
• Patients ≤30 kg body weight: Initiate at 5 mg daily and titrate as tolerated
up to 20 mg daily ( 2.1)
• Patients >30 kg body weight: Initiate at 10 mg daily and titrate as
tolerated up to 40 mg daily ( 2.1)
• Dosage adjustment needed in following groups:
o Geriatric patients ( 2.4, 8.5)
o Known CYP2C19 poor metabolizers ( 2.5)
o Mild or moderate hepatic impairment; no information for severe hepatic
impairment ( 2.7, 8.8)
• Tablets: Administer whole, broken in half along the score, or crush and mix
in applesauce ( 2.3)
• Tablets: Can be taken with or without food ( 2.3)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Risks from Concomitant Use with Opioids
Inform patients and caregivers that potentially fatal additive effects may occur if clobazam is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider [seeWarnings and Precautions ( 5.1 ), Drug Interactions ( 7.1 )] .
Abuse, Misuse, and Addiction
Inform patients that the use of clobazam, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug [see Warnings and Precautions (5.2)and Drug Abuse and Dependence (9.2)].
Withdrawal Reactions
Advise patients or caregivers that abrupt withdrawal of AEDs may increase their risk of seizure. Inform patients that the continued use of clobazam may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of clobazam may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of clobazam may require a slow taper [see Warnings and Precautions (5.3)and Drug Abuse and Dependence (9.3)].
Somnolence or Sedation
Advise patients or caregivers to check with their healthcare provider before clobazam is taken with other CNS depressants such as other benzodiazepines, opioids, tricyclic antidepressants, sedating antihistamines, or alcohol [seeWarnings and Precautions ( 5.4, 5.5)].
If applicable, caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that clobazam does not affect them adversely (e.g., impair judgment, thinking or motor skills).
Hypersensitivity
Inform patients or caregivers that clobazam is contraindicated in patients with a history of hypersensitivity to the drug or its ingredients [seeWarnings and Precautions ( 5.6 )].
Interactions with Hormonal Contraceptives
Counsel women to also use non-hormonal methods of contraception when clobazam is used with hormonal contraceptives and to continue these alternative methods for 28 days after discontinuing clobazam to ensure contraceptive reliability [seeDrug Interactions ( 7.3 ), Clinical Pharmacology ( 12.3 )].
Serious Dermatological Reactions
Advise patients or caregivers that serious skin reactions have been reported in patients taking clobazam. Serious skin reactions, including SJS/TEN, may need to be treated in a hospital and may be life-threatening. If a skin reaction occurs while taking clobazam, patients or caregivers should consult with healthcare providers immediately [seeWarnings and Precautions ( 5.6 )].
DRESS/Multiorgan Hypersensitivity
Instruct patients and caregivers that a fever or rash associated with signs of other organ system involvement (e.g., lymphadenopathy, hepatic dysfunction) may be drug-related and should be reported to their healthcare provider immediately. Clobazam should be discontinued immediately if a serious hypersensitivity reaction is suspected [see Warnings and Precautions (5.7)].
Suicidal Thinking and Behavior
Counsel patients, their caregivers, and their families that AEDs, including clobazam, may increase the risk of suicidal thoughts and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts of self-harm. Patients should report behaviors of concern immediately to healthcare providers [seeWarnings and Precautions (5.8)].
Pregnancy
Advise pregnant females that the use of clobazam late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns [ see Warnings and Precautions (5.9) and Use in Specific Populations (8.1)]. Instruct patients to notify their healthcare provider if they are pregnant.
Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant while taking clobazam. The registry is collecting information about the safety of antiepileptic drugs during pregnancy [ see Use in Specific Populations (8.1)].
Lactation
Counsel patients that clobazam, the active ingredient in clobazam tablet, is excreted in breast milk. Instruct patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients who have been administered clobazam to observe their infants for sedation, poor feeding and poor weight gain, and to seek medical attention if they notice these signs [see Use in Specific Populations (8.2)] .
Manufactured by:
Micro Labs Limited
Goa- 403 722, INDIA.
Manufactured for:
Micro Labs USA, Inc.
Somerset, NJ 08873
Rev. 03/2024
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AEDs, such as clobazam, during pregnancy. Healthcare providers are encouraged to recommend that pregnant women taking clobazam enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling 1-888-233-2334 or online at http://www.aedpregnancyregistry.org/.
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal [ see Warnings and Precautions (5.9) and Clinical Considerations ]. Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects ( see Data).
Administration of clobazam to pregnant rats and rabbits during the period of organogenesis or to rats throughout pregnancy and lactation resulted in developmental toxicity, including increased incidences of fetal malformations and mortality, at plasma exposures for clobazam and its major active metabolite, N-desmethylclobazam, below those expected at therapeutic doses in patients [see Animal Data]. Data for other benzodiazepines suggest the possibility of long-term effects on neurobehavioral and immunological function in animals following prenatal exposure to benzodiazepines at clinically relevant doses. Clobazam should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Advise a pregnant woman and women of childbearing age of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia, and sedation in neonates. Monitor neonates exposed to clobazam during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to clobazam during pregnancy for signs of withdrawal. Manage these neonates accordingly [ see Warnings and Precautions (5.9)].
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal Data
In a study in which clobazam (0, 150, 450, or 750 mg/kg/day) was orally administered to pregnant rats throughout the period of organogenesis, embryofetal mortality and incidences of fetal skeletal variations were increased at all doses. The low-effect dose for embryofetal developmental toxicity in rats (150 mg/kg/day) was associated with plasma exposures (AUC) for clobazam and its major active metabolite, N-desmethylclobazam, lower than those in humans at the maximum recommended human dose (MRHD) of 40 mg/day.
Oral administration of clobazam (0, 10, 30, or 75 mg/kg/day) to pregnant rabbits throughout the period of organogenesis resulted in decreased fetal body weights, and increased incidences of fetal malformations (visceral and skeletal) at the mid and high doses, and an increase in embryofetal mortality at the high dose. Incidences of fetal variations were increased at all doses. The highest dose tested was associated with maternal toxicity (ataxia and decreased activity). The low-effect dose for embryofetal developmental toxicity in rabbits (10 mg/kg/day) was associated with plasma exposures for clobazam and N-desmethylclobazam lower than those in humans at the MRHD.
Oral administration of clobazam (0, 50, 350, or 750 mg/kg/day) to rats throughout pregnancy and lactation resulted in increased embryofetal mortality at the high dose, decreased pup survival at the mid and high doses and alterations in offspring behavior (locomotor activity) at all doses. The low- effect dose for adverse effects on pre- and postnatal development in rats (50 mg/kg/day) was associated with plasma exposures for clobazam and N-desmethylclobazam lower than those in humans at the MRHD.
8.2 Lactation
Risk Summary
Clobazam is excreted in human milk ( see Data). There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. There are no data on the effects of clobazam on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for clobazam and any potential adverse effects on the breastfed infant from clobazam or from the underlying maternal condition.
Clinical Considerations
Adverse reactions such as somnolence and difficulty feeding have been reported in infants during breastfeeding in postmarketing experience with clobazam. Infants exposed to clobazam through breast milk should be monitored for sedation, poor feeding and poor weight gain.
Data
Scientific literature on clobazam use during lactation is limited. After short-term administration, clobazam and N-desmethylclobazam are transferred into breast milk.
8.3 Females and Males of Reproductive Potential
Administration of clobazam to rats prior to and during mating and early gestation resulted in adverse effects on fertility and early embryonic development at plasma exposures for clobazam and its major active metabolite, N-desmethylclobazam, below those in humans at the MRHD [see Nonclinical Toxicology (13.1)] .
8.4 Pediatric Use
Safety and effectiveness in patients less than 2 years of age have not been established.
In a study in which clobazam (0, 4, 36, or 120 mg/kg/day) was orally administered to rats during the juvenile period of development (postnatal days 14 to 48), adverse effects on growth (decreased bone density and bone length) and behavior (altered motor activity and auditory startle response; learning deficit) were observed at the high dose. The effect on bone density, but not on behavior, was reversible when drug was discontinued. The no-effect level for juvenile toxicity (36 mg/kg/day) was associated with plasma exposures (AUC) to clobazam and its major active metabolite, N-desmethylclobazam, less than those expected at therapeutic doses in pediatric patients.
8.5 Geriatric Use
Clinical studies of clobazam did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, elderly subjects appear to eliminate clobazam more slowly than younger subjects based on population pharmacokinetic analysis. For these reasons, the initial dose in elderly patients should be 5 mg/day. Patients should be titrated initially to 10 to 20 mg/day. Patients may be titrated further to a maximum daily dose of 40 mg if tolerated [seeDosage and Administration (2.4), Clinical Pharmacology (12.3)] .
8.6 CYP2C19 Poor Metabolizers
Concentrations of clobazam’s active metabolite, N-desmethylclobazam, are higher in CYP2C19 poor metabolizers than in extensive metabolizers. For this reason, dosage modification is recommended [seeDosage and Administration (2.5),Clinical Pharmacology (12.3)] .
8.7 Renal Impairment
The pharmacokinetics of clobazam were evaluated in patients with mild and moderate renal impairment. There were no significant differences in systemic exposure (AUC and C max) between patients with mild or moderate renal impairment and healthy subjects. No dose adjustment is required for patients with mild and moderate renal impairment. There is essentially no experience with clobazam in patients with severe renal impairment or ESRD. It is not known if clobazam or its active metabolite, N-desmethylclobazam, is dialyzable [seeDosage and Administration (2.6), Clinical Pharmacology (12.3)] .
8.8 Hepatic Impairment
Clobazam is hepatically metabolized; however, there are limited data to characterize the effect of hepatic impairment on the pharmacokinetics of clobazam. For this reason, dosage adjustment is recommended in patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9). There is inadequate information about metabolism of clobazam in patients with severe hepatic impairment [seeDosage and Administration (2.7), Clinical Pharmacology (12.3)] .
Pregnancy: Based on animal data, may cause fetal harm ( 8.1)
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Clobazam tablet contains clobazam, a Schedule IV controlled substance.
9.2 Abuse
Clobazam is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders [see Warnings and Precautions (5.2)].
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
The World Health Organization epidemiology database contains reports of drug abuse, misuse, and overdoses associated with clobazam.
9.3 Dependence
Physical Dependence
Clobazam may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use [see Warnings and Precautions (5.3)]. In clinical trials, cases of dependency were reported following abrupt discontinuation of clobazam.
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clobazam or reduce the dosage [see Dosage and Administration (2.2)and Warnings and Precautions (5.3)].
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to clobazam may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of clobazam may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
OVERDOSAGE SECTION
10 OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [ see Warnings and Precautions (5.2)]. Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway maintenance. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of action for clobazam, a 1,5-benzodiazepine, is not fully understood but is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABA Areceptor.
12.2 Pharmacodynamics
Effects on Electrocardiogram
The effect of clobazam 20 mg and 80 mg administered twice daily on QTc interval was evaluated in a randomized, evaluator-blinded, placebo-, and active-controlled (moxifloxacin 400 mg) parallel thorough QT study in 280 healthy subjects. In a study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo-adjusted, baseline-corrected QTc based on the Fridericia correction method was below 10 ms, the threshold for regulatory concern. Thus, at a dose two times the maximum recommended dose, clobazam did not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
The peak plasma levels (C max) and the area under the curve (AUC) of clobazam are dose-proportional over the dose range of 10 to 80 mg following single- or multiple-dose administration of clobazam. Based on a population pharmacokinetic analysis, the pharmacokinetics of clobazam are linear from 5 to 160 mg/day. Clobazam is converted to N-desmethylclobazam which has about 1/5 the activity of clobazam. The estimated mean elimination half-lives (t½) of clobazam and N-desmethylclobazam were 36 to 42 hours and 71 to 82 hours, respectively.
Absorption
Clobazam is rapidly and extensively absorbed following oral administration. The time to peak concentrations (T max) of clobazam tablets under fasted conditions ranged from 0.5 to 4 hours after single- or multiple-dose administrations. The relative bioavailability of clobazam tablets compared to an oral solution is approximately 100%. After single dose administration of the oral suspension under fasted conditions, the T maxranged from 0.5 to 2 hours. Based on exposure (C maxand AUC) of clobazam, clobazam tablets and suspension were shown to have similar bioavailability under fasted conditions. The administration of clobazam tablets with food or when crushed in applesauce does not affect absorption.
Although not studied, the oral bioavailability of the oral suspension is unlikely to be affected under fed conditions.
Distribution
Clobazam is lipophilic and distributes rapidly throughout the body. The apparent volume of distribution at steady state was approximately 100 L. The in vitroplasma protein binding of clobazam and N-desmethylclobazam is approximately 80 to 90% and 70%, respectively.
Metabolism and Excretion
Clobazam is extensively metabolized in the liver, with approximately 2% of the dose recovered in urine and 1% in feces as unchanged drug. The major metabolic pathway of clobazam involves N-demethylation, primarily by CYP3A4 and to a lesser extent by CYP2C19 and CYP2B6. N-desmethylclobazam, an active metabolite, is the major circulating metabolite in humans, and at therapeutic doses, plasma concentrations are 3 to 5 times higher than those of the parent compound. Based on animal and in vitroreceptor binding data, estimates of the relative potency of N-desmethylclobazam compared to parent compound range from 1/5 to equal potency. N-desmethylclobazam is extensively metabolized, mainly by CYP2C19. N-desmethylclobazam and its metabolites comprise ~94% of the total drug-related components in urine. Following a single oral dose of radiolabeled drug, approximately 11% of the dose was excreted in the feces and approximately 82% was excreted in the urine.
The polymorphic CYP2C19 is the major contributor to the metabolism of the pharmacologically active N -desmethylclobazam [seeClinical Pharmacology ( 12.5 )] . In CYP2C19 poor metabolizers, levels of N-desmethylclobazam were 5-fold higher in plasma and 2- to 3-fold higher in the urine than in CYP2C19 extensive metabolizers.
Pharmacokinetics in Specific Populations
Age
Population pharmacokinetic analyses showed that the clearance of clobazam is lower in elderly subjects compared to other age groups (ages 2 to 64). Dosing should be adjusted in the elderly [seeDosage and Administration ( 2.4 )] .
Sex
Population pharmacokinetic analyses showed no difference in the clearance of clobazam between women and men.
Race
Population pharmacokinetic analyses including Caucasian (75%), African American (15%), and Asian (9%) subjects showed that there is no evidence of clinically significant effect of race on the clearance of clobazam.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of clobazam was evaluated in patients with mild (creatinine clearance [CL CR] >50 to 80 mL/min; N=6) and moderate (CL CR=30 to 50 mL/min; N=6) renal dysfunction, with matching healthy controls (N=6), following administration of multiple doses of clobazam 20 mg/day. There were insignificant changes in C max(3 to 24%) and AUC (≤13%) for clobazam or N-desmethylclobazam in patients with mild or moderate renal impairment compared to patients with normal renal function. Patients with severe renal impairment or ESRD were not included in this study.
Hepatic Impairment
There are limited data to characterize the effect of hepatic impairment on the pharmacokinetics of clobazam. In a small study, the pharmacokinetics of a 20 mg single oral dose of clobazam in 9 patients with liver impairment were compared to healthy controls (N=6). The C maxand the mean plasma clearance of clobazam, as well as the C maxof N-desmethylclobazam, showed no significant change compared to the healthy controls. The AUC values of N-desmethylclobazam in these patients were not available. Adjust dosage in patients with hepatic impairment [seeDosage and Administration ( 2.7 )] .
Drug Interaction Studies
In vitro studies:
Clobazam did not inhibit CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, UGT1A1, UGT1A4, UGT1A6, or UGT2B4 in vitro. N-desmethylclobazam showed weak inhibition of CYP2C9, UGT1A4, UGT1A6 and UGT2B4.
Clobazam and N-desmethylclobazam did not significantly increase CYP1A2 or CYP2C19 activities, but did induce CYP3A4 activity in a concentration- dependent manner. Clobazam and N-desmethylclobazam also increased UGT1A1 mRNA but at concentrations much higher than therapeutic levels. The potential for clobazam or N-desmethylclobazam to induce CYP2B6 and CYP2C8 has not been evaluated.
Clobazam and N-desmethylclobazam do not inhibit P-glycoprotein (P-gp), but are P-gp substrates.
In vivo studies:
Potential for Clobazam to Affect Other Drugs
The effect of repeated 40 mg once-daily doses of clobazam on the pharmacokinetic profiles of single-dose dextromethorphan (CYP2D6 substrate), midazolam (CYP3A4 substrate), caffeine (CYP1A2 substrate), and tolbutamide (CYP2C9 substrate), was studied when these probe substrates were given as a drug cocktail (N=18).
Clobazam increased AUC and C maxof dextromethorphan by 90% and 59%, respectively, reflecting its inhibition of CYP2D6 in vivo. Drugs metabolized by CYP2D6 may require dose adjustment when used with clobazam.
Clobazam decreased the AUC and C maxof midazolam by 27% and 24%, respectively, and increased the AUC and C maxof the metabolite 1-hydroxymidazolam by 4-fold and 2-fold, respectively. This level of induction does not call for dosage adjustment of drugs that are primarily metabolized by CYP3A4 when used concomitantly with clobazam. Some hormonal contraceptives are metabolized by CYP3A4 and their effectiveness may be diminished when given with clobazam [seeDrug Interactions ( 7.3 )] . Repeated clobazam doses had no effect on caffeine and tolbutamide.
A population pharmacokinetic analysis indicated clobazam did not affect the exposure of valproic acid (a CYP2C9/2C19 substrate) or lamotrigine (a UGT substrate).
Potential for Other Drugs to Affect Clobazam
Co-administration of ketoconazole (a strong CYP3A4 inhibitor) 400 mg once- daily for 5 days increased clobazam AUC by 54%, with an insignificant effect on clobazam C max. There was no significant change in AUC and C maxof N-desmethylclobazam (N=18).
Strong (e.g., fluconazole, fluvoxamine, ticlopidine) and moderate (e.g., omeprazole) inhibitors of CYP2C19 may result in up to a 5-fold increase in exposure to N-desmethylclobazam, the active metabolite of clobazam, based on extrapolation from pharmacogenomic data [see Clinical Pharmacology (12.5)] . Dosage adjustment of clobazam may be necessary when co-administered with strong or moderate CYP2C19 inhibitors [seeDrug Interactions ( 7.4 )] .
The effects of concomitant antiepileptic drugs that are CYP3A4 inducers (phenobarbital, phenytoin, and carbamazepine), CYP2C19 inducers (valproic acid, phenobarbital, phenytoin, and carbamazepine), and CYP2C19 inhibitors (felbamate and oxcarbazepine) were evaluated using data from clinical trials. Results of population pharmacokinetic analysis show that these concomitant antiepileptic drugs did not significantly alter the pharmacokinetics of clobazam or N-desmethylclobazam at steady-state.
Alcohol has been reported to increase the maximum plasma exposure of clobazam by approximately 50%. Alcohol may have additive CNS depressant effects when taken with clobazam [seeWarnings and Precautions ( 5.4 ), Drug Interactions ( 7.2 )] .
12.5 Pharmacogenomics
The polymorphic CYP2C19 is the main enzyme that metabolizes the pharmacologically active N-desmethylclobazam. Compared to CYP2C19 extensive metabolizers, N-desmethylclobazam AUC and C maxare approximately 3 to 5 times higher in poor metabolizers (e.g., subjects with *2/*2 genotype) and 2 times higher in intermediate metabolizers (e.g., subjects with *1/*2 genotype). The prevalence of CYP2C19 poor metabolism differs depending on racial/ethnic background. Dosage in patients who are known CYP2C19 poor metabolizers may need to be adjusted [seeDosage and Administration (2.5)] .
The systemic exposure of clobazam is similar for both CYP2C19 poor and extensive metabolizers.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In mice, oral administration of clobazam (0, 6, 12, or 24 mg/kg/day) for 2 years did not result in an increase in tumors. The highest dose tested was approximately 3 times the maximum recommended human dose (MRHD) of 40 mg/day, based on body surface area (mg/m 2).
In rats, oral administration of clobazam for 2 years resulted in increases in tumors of the thyroid gland (follicular cell adenoma and carcinoma) and liver (hepatocellular adenoma) at the mid and high doses. The low dose, not associated with an increase in tumors, was associated with plasma exposures (AUC) for clobazam and its major active metabolite, N-desmethylclobazam, less than that in humans at the MRHD.
Mutagenesis
Clobazam and the major active metabolite, N-desmethylclobazam, were negative for genotoxicity, based on data from a battery of in vitro(bacteria reverse mutation, mammalian clastogenicity) and in vivo(mouse micronucleus) assays.
Impairment of Fertility
In a fertility study in which clobazam (50, 350, or 750 mg/kg/day, corresponding to 12, 84 and 181 times the oral Maximum Recommended Human Dose, MRHD, of 40 mg/day based on mg/m 2body surface) was orally administered to male and female rats prior to and during mating and continuing in females to gestation day 6, increases in abnormal sperm and pre-implantation loss were observed at the highest dose tested. The no-effect level for fertility and early embryonic development in rats was associated with plasma exposures (AUC) for clobazam and its major active metabolite, N-desmethylclobazam, less than those in humans at the maximum recommended human dose of 40 mg/day.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Clobazam Tablets, 10 mg are white to off-white, oval shaped tablets with a functional score line on one face and "1" and "0" debossed on the other face. Each tablet contains 10 mg of clobazam.
Bottles of 30 NDC 42571-315-30
Bottles of 90 NDC 42571-315-90
Bottles of 100 NDC 42571-315-01
Bottles of 500 NDC 42571-315-05
Carton of (Alu-Alu blister) 100 (10 x 10) Unit-dose Tablets NDC 42571-315-11
Carton of (Alu-PVC/ACLAR blister) 100 (10 x 10) Unit-dose Tablets NDC 42571-315-91
Clobazam Tablets, 20 mg are white to off-white, oval shaped tablets with a functional score line on one face and "2" and "0" debossed on the other face. Each tablet contains 20 mg of clobazam.
Bottles of 30 NDC 42571-316-30
Bottles of 90 NDC 42571-316-90
Bottles of 100 NDC 42571-316-01
Carton of (Alu-Alu blister) 100 (10 x 10) Unit-dose Tablets NDC 42571-316-11
Carton of (Alu-PVC/ACLAR blister) 100 (10 x 10) Unit-dose Tablets NDC 42571-316-91
Store tablets at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
