Irbesartan and Hydrochlorothiazide
These highlights do not include all the information needed to use IRBESARTAN AND HYDROCHLOROTHIAZIDE TABLETS safely and effectively. See full prescribing information for IRBESARTAN AND HYDROCHLOROTHIAZIDE TABLETS.IRBESARTAN AND HYDROCHLOROTHIAZIDE tablets, for oral useInitial U.S. Approval: 1997
599548e1-5879-4160-871a-b8c3f6e19652
HUMAN PRESCRIPTION DRUG LABEL
Jan 1, 2023
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Irbesartan and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Irbesartan and Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
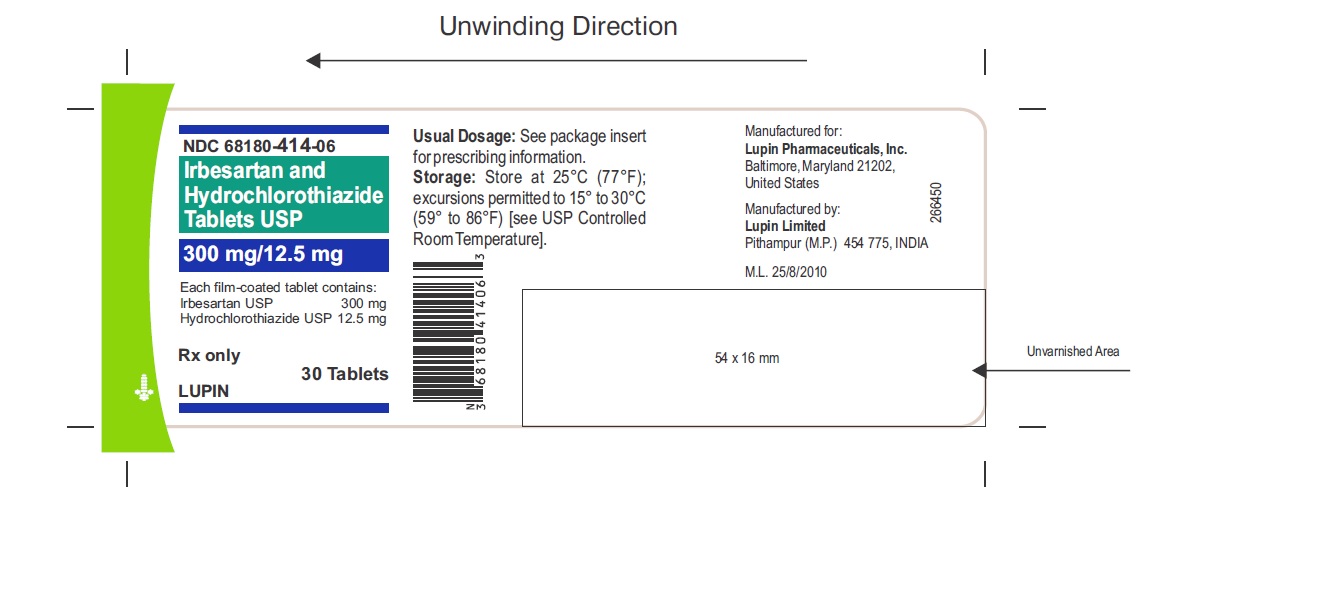
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Irbesartan and Hydrochlorothiazide Tablets USP
Rx Only
150 mg/12.5 mg
NDC 68180-413-06
30’s Tablets

Irbesartan and Hydrochlorothiazide Tablets USP
Rx Only
300 mg/12.5 mg
NDC 68180-414-06
30’s Tablets
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
- Irbesartan and hydrochlorothiazide is contraindicated in patients who are hypersensitive to any component of this product.
- Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
- Do not coadminister aliskiren with irbesartan and hydrochlorothiazide in patients with diabetes [see Drug Interactions(7)].
- Hypersensitivity to any component of this product. (4)
- Anuria. (4)
- Hypersensitivity to sulfonamide-derived drugs (4)
- Do not coadminister aliskiren with irbesartan and hydrochlorothiazide in patients with diabetes (4)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Nonsteroidal Anti-inflammatory Agents Including Selective
Cyclooxygenase-2 Inhibitors (COX- 2 Inhibitors)
Irbesartan
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including irbesartan, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Therefore, monitor renal function and blood pressure periodically in patients receiving irbesartan and NSAID therapy.
Hydrochlorothiazide
Administration of a nonsteroidal anti-inflammatory agent, including a selective COX-2 inhibitor can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing, and thiazide diuretics. Therefore, when irbesartan and hydrochlorothiazide tablets and nonsteroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
7.2 Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin-receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function, and electrolytes in patients on irbesartan and hydrochlorothiazide and other agents that affect the RAS.
In most patients no benefit has been associated with using two RAS inhibitors concomitantly. In general, avoid combined use of RAS inhibitors.
Do not coadminister aliskiren with irbesartan and hydrochlorothiazide in patients with diabetes. Avoid use of aliskiren with irbesartan and hydrochlorothiazide in patients with renal impairment (GFR < 60 mL/min).
7.3 Agents Increasing Serum Potassium
Coadministration of irbesartan and hydrochlorothiazide tablets with other drugs that raise serum potassium levels may result in hyperkalemia, sometimes severe. Monitor serum potassium in such patients.
7.4 Antidiabetic Drugs (oral agents and insulin)
Dosage adjustment of the antidiabetic drug may be required when coadministered with hydrochlorothiazide.
7.5 Cholestyramine and Colestipol Resins
Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Stagger the dosage of hydrochlorothiazide and the resin such that irbesartan and hydrochlorothiazide is administered at least 4 hours before or 4 to 6 hours after the administration of the resin.
7.6 Lithium
Increases in serum lithium concentrations and lithium toxicity have been reported with concomitant use of irbesartan or thiazide diuretics. Monitor lithium levels in patients receiving irbesartan and hydrochlorothiazide and lithium.
7.7 Carbamazepine
Concomitant use of carbamazepine and hydrochlorothiazide has been associated with the risk of symptomatic hyponatremia. Monitor electrolytes during concomitant use.
- NSAIDs and selective COX-2 inhibitors: Can reduce diuretic, natriuretic of diuretic, may lead to increased risk of renal impairment and reduced antihypertensive effect. Monitor renal function periodically. (7)
- Dual blockade of the renin-angiotensin system: Increased risk of renal impairment, hypotension, and hyperkalemia. (7)
- Antidiabetic drugs: Dosage adjustment of antidiabetic may be required. (7)
- Cholestyramine and colestipol: Reduced absorption of thiazides. (7)
- Lithium: Increases in serum lithium concentrations and lithium toxicity. (7)
- Carbamazepine: Increased risk of hyponatremia. (7)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
The side effects of irbesartan are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. [see Adverse Reactions (6)].
Maximum antihypertensive effects are attained within 2 to 4 weeks after a change in dose.
Irbesartan and hydrochlorothiazide tablets may be administered with or without food.
Irbesartan and hydrochlorothiazide tablets may be administered with other antihypertensive agents.
Renal Impairment
The usual regimens of therapy with irbesartan and hydrochlorothiazide tablets may be followed as long as the patient's creatinine clearance is >30 mL/min. In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so irbesartan and hydrochlorothiazide is not recommended.
Hepatic Impairment
No dosage adjustment is necessary in patients with hepatic impairment.
2.2 Add-On Therapy
In patients not controlled on monotherapy with irbesartan or hydrochlorothiazide, the recommended doses of irbesartan and hydrochlorothiazide tablets, in order of increasing mean effect, are (irbesartan and hydrochlorothiazide) 150/12.5 mg, 300/12.5 mg, and 300/25 mg. The largest incremental effect will likely be in the transition from monotherapy to 150/12.5 mg. [see Clinical Studies (14.2)].
2.3 Replacement Therapy
Irbesartan and hydrochlorothiazide tablets may be substituted for the titrated components.
2.4 Initial Therapy
The usual starting dose is irbesartan and hydrochlorothiazide tablets, 150/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of 300/25 mg once daily as needed to control blood pressure [see Clinical Studies (14.2)]. Irbesartan and hydrochlorothiazide tablet is not recommended as initial therapy in patients with intravascular volume depletion [see Warnings and Precautions (5.2)].
General Considerations
- Maximum effects within 2 to 4 weeks after dose change. (2.1)
- Renal impairment: Not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min). (2.1, 5.8)
Hypertension
- Initiate with 150/12.5 mg. Titrate to 300/12.5 mg then 300/25 mg if needed. (2.2)
- Replacement therapy: May be substituted for titrated components. (2.3)
DESCRIPTION SECTION
11 DESCRIPTION
Irbesartan and hydrochlorothiazide tablets USP are a combination of an angiotensin II receptor antagonist (AT1 subtype), irbesartan, and a thiazide diuretic, hydrochlorothiazide (HCTZ).
Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and its structural formula is:
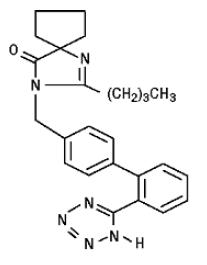
Irbesartan is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
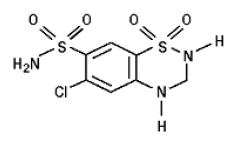
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water and freely soluble in sodium hydroxide solution.
Irbesartan and hydrochlorothiazide tablets USP are available for oral administration in film-coated tablets containing either 150 mg or 300 mg of irbesartan combined with 12.5 mg of hydrochlorothiazide. All dosage strengths contain the following inactive ingredients: colloidal silicon dioxide, crospovidone, ferric oxide black, ferric oxide red, ferric oxide yellow, hypromellose-2910, magnesium stearate, mannitol, poloxamer 188, polyethylene glycol-3350, povidone, propylene glycol, shellac and titanium dioxide.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Irbesartan Monotherapy
The antihypertensive effects of irbesartan were examined in 7 major placebo- controlled, 8 to 12-week trials in patients with baseline diastolic blood pressures of 95 to 110 mmHg. Doses of 1 to 900 mg were included in these trials in order to fully explore the dose-range of irbesartan. These studies allowed a comparison of once or twice-daily regimens at 150 mg/day, comparisons of peak and trough effects, and comparisons of response by gender, age, and race. Two of the 7 placebo-controlled trials identified above and 2 additional placebo-controlled studies examined the antihypertensive effects of irbesartan and hydrochlorothiazide in combination.
The 7 studies of irbesartan monotherapy included a total of 1915 patients randomized to irbesartan (1 to 900 mg) and 611 patients randomized to placebo. Once-daily doses of 150 to 300 mg provided statistically and clinically significant decreases in systolic and diastolic blood pressure with trough (24-hour post dose) effects after 6 to 12 weeks of treatment compared to placebo, of about 8 to 10/5 to 6 mmHg and 8 to 12/5 to 8 mmHg, respectively. No further increase in effect was seen at dosages greater than 300 mg. The dose-response relationships for effects on systolic and diastolic pressure are shown in Figures 3 and 4.
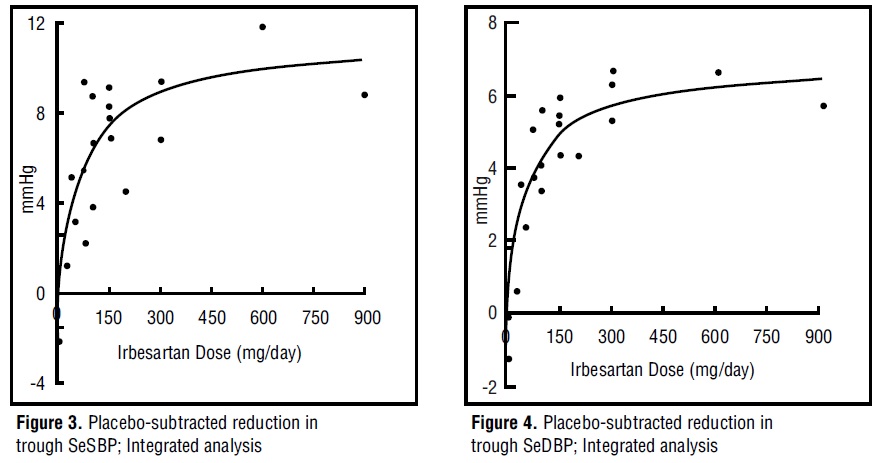
Once-daily administration of therapeutic doses of irbesartan gave peak effects at around 3 to 6 hours and, in one continuous ambulatory blood pressure monitoring study, again around 14 hours. This was seen with both once-daily and twice-daily dosing. Trough-to-peak ratios for systolic and diastolic response were generally between 60% and 70%. In a continuous ambulatory blood pressure monitoring study, once-daily dosing with 150 mg gave trough and mean 24-hour responses similar to those observed in patients receiving twice-daily dosing at the same total daily dose.
Analysis of age, gender, and race subgroups of patients showed that men and women, and patients over and under 65 years of age, had generally similar responses. Irbesartan was effective in reducing blood pressure regardless of race, although the effect was somewhat less in blacks (usually a low-renin population). Black patients typically show an improved response with the addition of a low dose diuretic (e.g., 12.5 mg hydrochlorothiazide).
The effect of irbesartan is apparent after the first dose and is close to the full observed effect at 2 weeks. At the end of the 8-week exposure, about 2/3 of the antihypertensive effect was still present 1 week after the last dose. Rebound hypertension was not observed. There was essentially no change in average heart rate in irbesartan-treated patients in controlled trials.
14.2 Irbesartan and Hydrochlorothiazide
The antihypertensive effects of irbesartan and hydrochlorothiazide tablets were examined in 4 placebo-controlled studies in patients with mild-moderate hypertension (mean seated diastolic blood pressure [SeDBP] between 90 and 110 mmHg), one study in patients with moderate hypertension (mean seated systolic blood pressure [SeSBP] 160 to 179 mmHg or SeDBP 100 to 109 mmHg), and one study in patients with severe hypertension (mean SeDBP ≥110 mmHg) of 8 to 12 weeks. These trials included 3149 patients randomized to fixed doses of irbesartan (37.5 to 300 mg) and concomitant hydrochlorothiazide (6.25 to 25 mg).
Study I was a factorial study that compared all combinations of irbesartan (37.5 mg, 100 mg, and 300 mg or placebo) and hydrochlorothiazide (6.25 mg, 12.5 mg, and 25 mg or placebo).
Study II compared the irbesartan and hydrochlorothiazide combinations of 75/12.5 mg and 150/12.5 mg to their individual components and placebo.
Study III investigated the ambulatory blood pressure responses to irbesartan and hydrochlorothiazide (75/12.5 mg and 150/12.5 mg) and placebo after 8 weeks of dosing.
Study IV investigated the effects of the addition of irbesartan (75 or 150 mg) in patients not controlled (SeDBP 93 to 120 mmHg) on hydrochlorothiazide (25 mg) alone. In Studies I to III, the addition of irbesartan 150 to 300 mg to hydrochlorothiazide doses of 6.25, 12.5, or 25 mg produced further dose- related reductions in blood pressure at trough of 8 to 10 mmHg/3 to 6 mmHg, similar to those achieved with the same monotherapy dose of irbesartan. The addition of hydrochlorothiazide to irbesartan produced further dose-related reductions in blood pressure at trough (24 hours post-dose) of 5 to 6/2 to 3 mmHg (12.5 mg) and 7 to 11/4 to 5 mmHg (25 mg), also similar to effects achieved with hydrochlorothiazide alone. Once-daily dosing with 150 mg irbesartan and 12.5 mg hydrochlorothiazide, 300 mg irbesartan and 12.5 mg hydrochlorothiazide, or 300 mg irbesartan and 25 mg hydrochlorothiazide produced mean placebo-adjusted blood pressure reductions at trough (24 hours post-dosing) of about 13 to 15/7 to 9 mmHg, 14/9 to 12 mmHg, and 19 to 21/11 to 12 mmHg, respectively. Peak effects occurred at 3 to 6 hours, with the trough-to-peak ratios >65%.
In Study IV, the addition of irbesartan (75 to 150 mg) gave an additive effect (systolic/diastolic) at trough (24 hours post-dosing) of 11/7 mmHg.
Initial Therapy
Studies V and VI had no placebo group, so effects described below are not all attributable to irbesartan or HCTZ.
Study V was conducted in patients with a mean baseline blood pressure of 162/98 mmHg and compared the change from baseline in SeSBP at 8 weeks between the combination group (irbesartan and HCTZ 150/12.5 mg), to irbesartan (150 mg) and to HCTZ (12.5 mg). These initial study regimens were increased at 2 weeks to irbesartan and hydrochlorothiazide 300/25 mg, irbesartan 300 mg, or to HCTZ 25 mg, respectively.
Mean reductions from baseline for SeDBP and SeSBP at trough were 14.6 mmHg and 27.1 mmHg for patients treated with irbesartan and hydrochlorothiazide, 11.6 mmHg and 22.1 mmHg for patients treated with irbesartan, and 7.3 mmHg and 15.7 mmHg for patients treated with HCTZ at 8 weeks, respectively. For patients treated with irbesartan and hydrochlorothiazide, the mean change from baseline in SeDBP was 3.0 mmHg lower (p=0.0013) and the mean change from baseline in SeSBP was 5.0 mmHg lower (p=0.0016) compared to patients treated with irbesartan, and 7.4 mmHg lower (p<0.0001) and 11.3 mmHg lower (p<0.0001) compared to patients treated with HCTZ, respectively. Withdrawal rates were 3.8% on irbesartan, 4.8% on HCTZ, and 6.7% on irbesartan and hydrochlorothiazide.
Study VI was conducted in patients with a mean baseline blood pressure of 172/113 mmHg and compared trough SeDBP at 5 weeks between the combination group (irbesartan and HCTZ 150/12.5 mg) and irbesartan (150 mg). These initial study regimens were increased at 1 week to irbesartan and hydrochlorothiazide 300/25 mg or to irbesartan 300 mg, respectively.
At 5 weeks, mean reductions from baseline for SeDBP and SeSBP at trough were 24.0 mmHg and 30.8 mmHg for patients treated with irbesartan and hydrochlorothiazide and 19.3 mmHg and 21.1 mmHg for patients treated with irbesartan, respectively. The mean SeDBP was 4.7 mmHg lower (p<0.0001) and the mean SeSBP was 9.7 mmHg lower (p<0.0001) in the group treated with irbesartan and hydrochlorothiazide than in the group treated with irbesartan. Patients treated with irbesartan and hydrochlorothiazide achieved more rapid blood pressure control with significantly lower SeDBP and SeSBP and greater blood pressure control at every assessment (Week 1, Week 3, Week 5, and Week 7). Maximum effects were seen at Week 7.
Withdrawal rates were 2.2% on irbesartan and 2.1% on irbesartan and hydrochlorothiazide.
In Studies I to VI, there was no difference in response for men and women or in patients over or under 65 years of age. Black patients had a larger response to hydrochlorothiazide than non-black patients and a smaller response to irbesartan. The overall response to the combination was similar for black and non-black patients.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Irbesartan and hydrochlorothiazide tablets USP are film-coated tablets, have markings on one side and available in the strengths and packages listed in the following table:
|
** Tablet Strength (irbesartan and hydrochlorothiazide)** |
** Film-Coated Tablet Color/Shape** |
** Tablet** |
** Package Size** |
** NDC Code** |
|
** 150 mg and 12.5 mg** |
light peach, biconvex, oval shaped |
Imprinted with "L058" on one side and plain on other side |
Bottles of 30 |
68180-413-06 |
|
** 300 mg and 12.5 mg** |
light peach, biconvex, oval shaped |
Imprinted with "L059" on one side and plain on other side |
Bottles of 30 |
68180-414-06 |
16.2 Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Irbesartan and Hydrochlorothiazide
No carcinogenicity studies have been conducted with the irbesartan and hydrochlorothiazide combination.
Irbesartan and hydrochlorothiazide was not mutagenic in standard in vitro tests (Ames microbial test and Chinese hamster mammalian-cell forward gene- mutation assay). Irbesartan and hydrochlorothiazide was negative in tests for induction of chromosomal aberrations (in vitro-human lymphocyte assay; in vivo-mouse micronucleus study).
The combination of irbesartan and hydrochlorothiazide has not been evaluated in definitive studies of fertility.
Irbesartan
No evidence of carcinogenicity was observed when irbesartan was administered at doses of up to 500/1000 mg/kg/day (males/females, respectively) in rats and 1000 mg/kg/day in mice for up to 2 years. For male and female rats, 500 mg/kg/day provided an average systemic exposure to irbesartan (AUC0 to 24 hours, bound plus unbound) about 3 and 11 times, respectively, the average systemic exposure in humans receiving the maximum recommended dose (MRHD) of 300 mg irbesartan/day, whereas 1000 mg/kg/day (administered to females only) provided an average systemic exposure about 21 times that reported for humans at the MRHD. For male and female mice, 1000 mg/kg/day provided an exposure to irbesartan about 3 and 5 times, respectively, the human exposure at 300 mg/day.
Irbesartan was not mutagenic in a battery of in vitro tests (Ames microbial test, rat hepatocyte DNA repair test, V79 mammalian-cell forward gene-mutation assay). Irbesartan was negative in several tests for induction of chromosomal aberrations (in vitro-human lymphocyte assay; in vivo-mouse micronucleus study).
Irbesartan had no adverse effects on fertility or mating of male or female rats at oral doses ≤650 mg/kg/day, the highest dose providing a systemic exposure to irbesartan (AUC0 to 24 hours, bound plus unbound) about 5 times that found in humans receiving the MRHD of 300 mg/day.
Hydrochlorothiazide
Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 µg/mL, and in the Aspergillus nidulans non-disjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to mating and throughout gestation.
