Kids Tummy Relief
Kids' Tummy Relief
a7831b84-1f51-9258-e053-2a95a90a901e
HUMAN OTC DRUG LABEL
May 23, 2025
Genexa Inc.
DUNS: 079751024
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Calcium carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
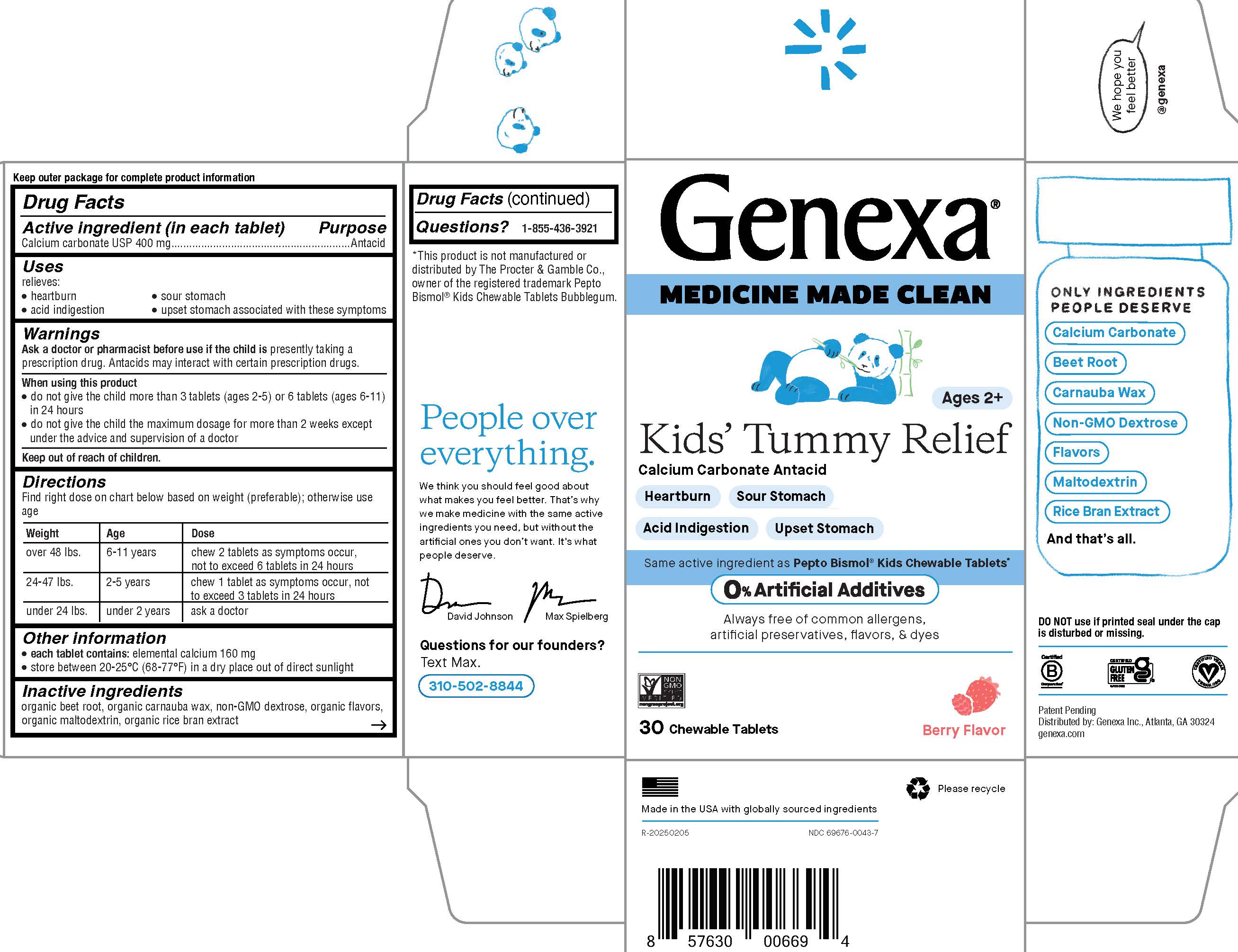
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Genexa**®**
MEDICINE MADE CLEAN
Ages 2+
Kids' Tummy Relief
Calcium Carbonate Antacid
Heartburn
Sour Stomach
Acid Indigestion
Upset Stomach
Same active ingredient asPepto Bismol****®**** Kids Chewable Tablets*
0% Artificial Additives
Always free of common allergens, artificial preservatives, flavors, & dyes
30 Chewable Tablets
Berry Flavor
INDICATIONS & USAGE SECTION
Uses
relieves:
- heartburn
- acid indigestion
- sour stomach
- upset stomach associated with these symptoms
SPL UNCLASSIFIED SECTION
Made in the USA with globally sourced ingredients
NDC 69676-0043-7
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each tablet)
Calcium carbonate USP 400 mg
OTC - PURPOSE SECTION
Purpose
Antacid
WARNINGS SECTION
Warnings
Ask a doctor or pharmacist before use if the child ispresently taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- do not give the child more than 3 tablets (ages 2-5) or 6 tablets (ages 6-11) in 24 hours
- do not give the child the maximum dosage for more than 2 weeks except under the advice and supervision of a doctor
Keep out of reach of children.
DOSAGE & ADMINISTRATION SECTION
Directions
Find right dose on chart below based on weight (preferable); otherwise use age
|
Weight |
Age |
Dose |
|
over 48 lbs. |
6-11 years |
chew 2 tablets as symptoms occur, not to exceed 6 tablets in 24 hours |
|
24-47 lbs. |
2-5 years |
chew 1 tablet as symptoms occur, not to exceed 3 tablets in 24 hours |
|
under 24 lbs. |
under 2 years |
ask a doctor |
INACTIVE INGREDIENT SECTION
Inactive ingredients
organic beet root, organic carnauba wax, non-GMO dextose, organic flavors, organic maltodextrin, organic rice bran extract
OTC - QUESTIONS SECTION
Questions?
1-855-436-3921
