Sunsolve MD Mineral Shade Amber 002
Sunsolve MD Mineral Shade Amber 002
Approved
Approval ID
26e00001-9c68-0734-e063-6394a90a1787
Product Type
HUMAN OTC DRUG LABEL
Effective Date
Aug 27, 2025
Manufacturers
FDA
Sunsolve MD Inc
DUNS: 119376976
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Zinc Oxide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code84878-402
Application NumberM020
Product Classification
M
Marketing Category
C200263
G
Generic Name
Zinc Oxide
Product Specifications
Route of AdministrationTOPICAL
Effective DateAugust 27, 2025
FDA Product Classification
INGREDIENTS (28)
TRILAURETH-4 PHOSPHATEInactive
Code: M96W2OLL2V
Classification: IACT
HEXYLENE GLYCOLInactive
Code: KEH0A3F75J
Classification: IACT
GLYCERINInactive
Code: PDC6A3C0OX
Classification: IACT
DIMETHICONOL/PROPYLSILSESQUIOXANE/SILICATE CROSSPOLYMER (450000000 MW)Inactive
Code: 9KB5R958PB
Classification: IACT
PROPANEDIOLInactive
Code: 5965N8W85T
Classification: IACT
TETRASODIUM GLUTAMATE DIACETATEInactive
Code: 5EHL50I4MY
Classification: IACT
BUTYLOCTYL SALICYLATEInactive
Code: 2EH13UN8D3
Classification: IACT
ETHYLHEXYLGLYCERINInactive
Code: 147D247K3P
Classification: IACT
OCTYLDODECYL NEOPENTANOATEInactive
Code: X8725R883T
Classification: IACT
ISODODECANEInactive
Code: A8289P68Y2
Classification: IACT
.ALPHA.-BISABOLOL, (+)-Inactive
Code: 105S6I733Z
Classification: IACT
ZINC OXIDEActive
Quantity: 132 mg in 1 mL
Code: SOI2LOH54Z
Classification: ACTIB
SODIUM HYDROXIDEInactive
Code: 55X04QC32I
Classification: IACT
WATERInactive
Code: 059QF0KO0R
Classification: IACT
NIACINAMIDEInactive
Code: 25X51I8RD4
Classification: IACT
SODIUM CHLORIDEInactive
Code: 451W47IQ8X
Classification: IACT
FERRIC OXIDE REDInactive
Code: 1K09F3G675
Classification: IACT
PHENOXYETHANOLInactive
Code: HIE492ZZ3T
Classification: IACT
POLYMETHYLSILSESQUIOXANE (4.5 MICRONS)Inactive
Code: 59Z907ZB69
Classification: IACT
LAURYL PEG-8 DIMETHICONE (300 CPS)Inactive
Code: ELL2U7K8T8
Classification: IACT
ALKYL (C12-15) BENZOATEInactive
Code: A9EJ3J61HQ
Classification: IACT
FERRIC OXIDE YELLOWInactive
Code: EX438O2MRT
Classification: IACT
ALLANTOINInactive
Code: 344S277G0Z
Classification: IACT
PEG-10Inactive
Code: 761NX2Q08Y
Classification: IACT
CAPRYLYL METHICONEInactive
Code: Q95M2P1KJL
Classification: IACT
DIMETHICONEInactive
Code: 92RU3N3Y1O
Classification: IACT
FERROSOFERRIC OXIDEInactive
Code: XM0M87F357
Classification: IACT
CAPRYLYL GLYCOLInactive
Code: 00YIU5438U
Classification: IACT
Drug Labeling Information
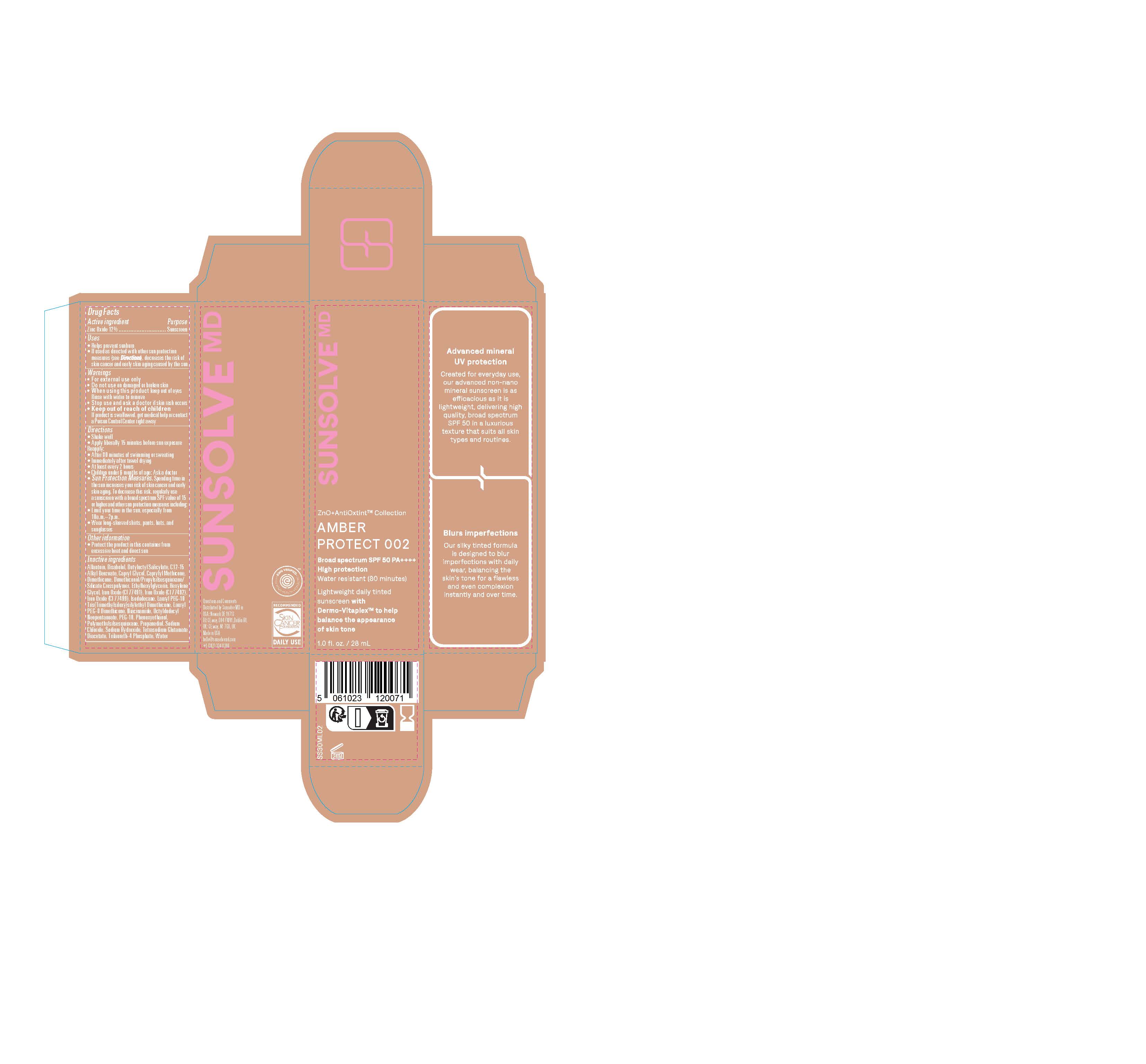
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 11/5/2024
Sunsolve MD Mineral Shade Amber 002
INDICATIONS & USAGE SECTION
LOINC: 34067-9Updated: 11/5/2024
Sunsolve MD Mineral Shade Amber 002

DOSAGE & ADMINISTRATION SECTION
LOINC: 34068-7Updated: 11/5/2024
Sunsolve MD Mineral Shade Amber 002

WARNINGS SECTION
LOINC: 34071-1Updated: 11/5/2024
Sunsolve MD Mineral Shade Amber 002

INACTIVE INGREDIENT SECTION
LOINC: 51727-6Updated: 11/5/2024
Sunsolve MD Mineral Shade Amber 002

OTC - KEEP OUT OF REACH OF CHILDREN SECTION
LOINC: 50565-1Updated: 11/5/2024
Sunsolve MD Mineral Shade Amber 002

OTC - PURPOSE SECTION
LOINC: 55105-1Updated: 11/5/2024
Sunsolve MD Mineral Shade Amber 002

OTC - ACTIVE INGREDIENT SECTION
LOINC: 55106-9Updated: 11/5/2024
Sunsolve MD Mineral Shade Amber 002

