BaoDao TATTOO NUMBING
84010-114
352571b6-c8f1-3d4c-e063-6394a90a83ab
HUMAN OTC DRUG LABEL
May 14, 2025
Jiangxi Hemei Pharmaceutical Co., Ltd
DUNS: 724892056
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lidocaine 4% TATTOO NUMBING
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
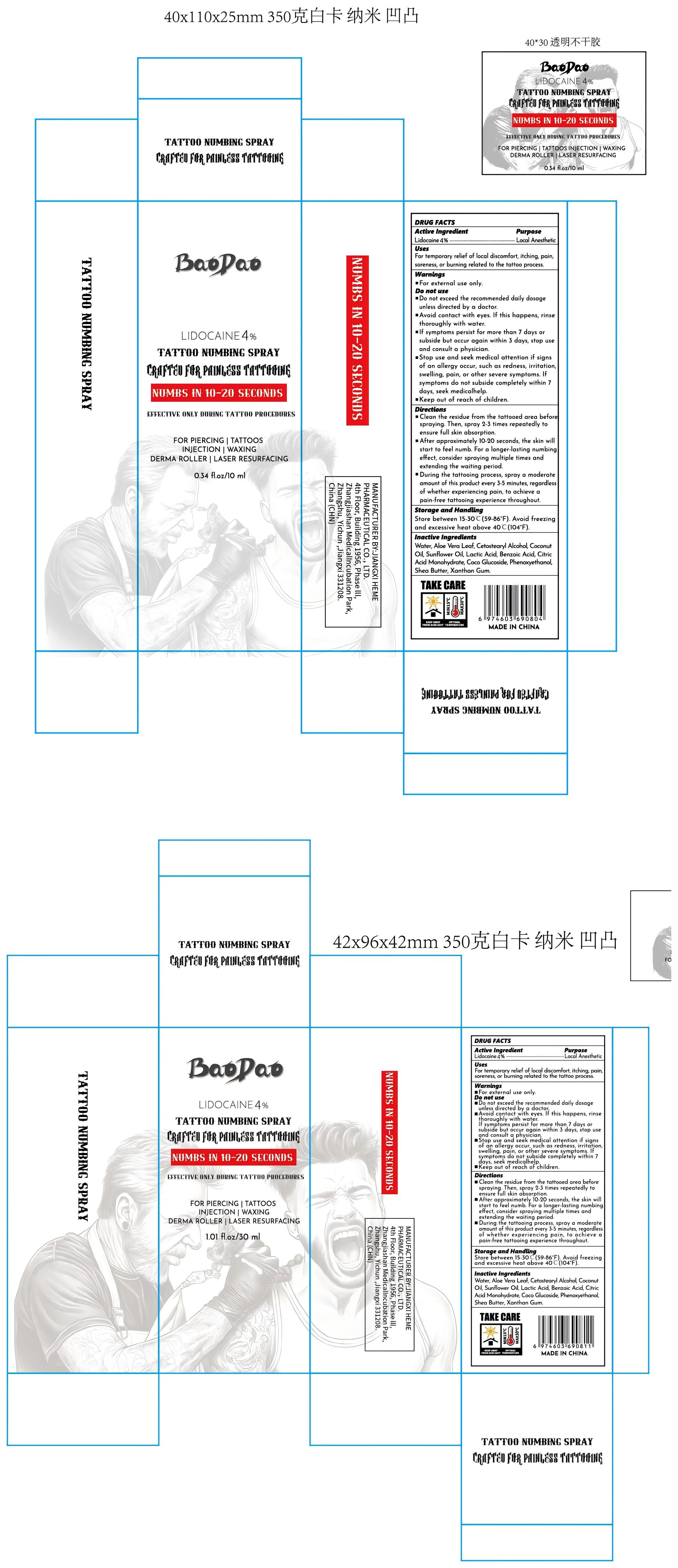
INDICATIONS & USAGE SECTION
Use
For temporary relief of local discomfort,itching, pain,soreness,or burning related to the tattoo process.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Lidocaine 4%
OTC - PURPOSE SECTION
Purpose
Local Anesthetic
WARNINGS SECTION
Warnings
For external use only.
OTC - DO NOT USE SECTION
Do not use
Do not exceed the recommended daily dosage unless directed by a doctor.
Avoid contact with eyes.If this happens, rinse thoroughly with water.
If symptoms persist for more than 7 days or subside but occur again within 3 days, stop use and consult a physician.
Stop use and seek medical attention if signs of an allergy occur, such as redness, irritation, swelling, pain, or other severe symptoms.If symptoms do not subside completely within 7 days, seek medicahelp.
OTC - WHEN USING SECTION
When Using
Avoid contact with eyes. If this happens, rinse thoroughly with water.
OTC - STOP USE SECTION
Stop Use
If symptoms persist for more than 7 days or subside but occur again within 3 days, stop use and consult a physician.
OTC - ASK DOCTOR SECTION
Ask Doctor
Stop use and seek medical attention if signs of an allergy occur, such as redness, irritation, swelling ,pain,or other severe symptoms.If symptoms do not subside completely within 7 days, seek medicalhelp.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out Of Reach Of Children
Keep out of reach of children.
DOSAGE & ADMINISTRATION SECTION
Directions
Clean the residue from the tattooed area before spraying.Then, spray 2-3 times repeatedly to ensure full skin absorption.
After approximately 10-20 seconds, the skin will start to feel numb. For a longer-lasting numbing effect, consider spraying multiple times and extending the waiting period.
During the tattooing process, spray a moderate amount of this product every 3-5 minutes, regardless of whether experiencing pain, to achieve a pain-free tattooing experience throughout.
STORAGE AND HANDLING SECTION
Other information
Store between 15-30℃(59-86°F). Avoid freezing and excessive heat above 40℃(104°F).
INACTIVE INGREDIENT SECTION
Inactive ingredients
Water,Aloe Vera Leaf,Cetostearyl Alcohol,CoconutOil,Sunfower Oil,Lactic Acid,Benzoic Acid,Citric Acid Monohydrate,Coco Glucoside,Phenoxyethanol,Shea Butter,Xanthan Gum.
