YVTYVT DARK SPOT
352699fa-f746-c931-e063-6394a90a6391
HUMAN OTC DRUG LABEL
May 15, 2025
Beijing JUNGE Technology Co., Ltd.
DUNS: 848718652
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DARK SPOT
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (21)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
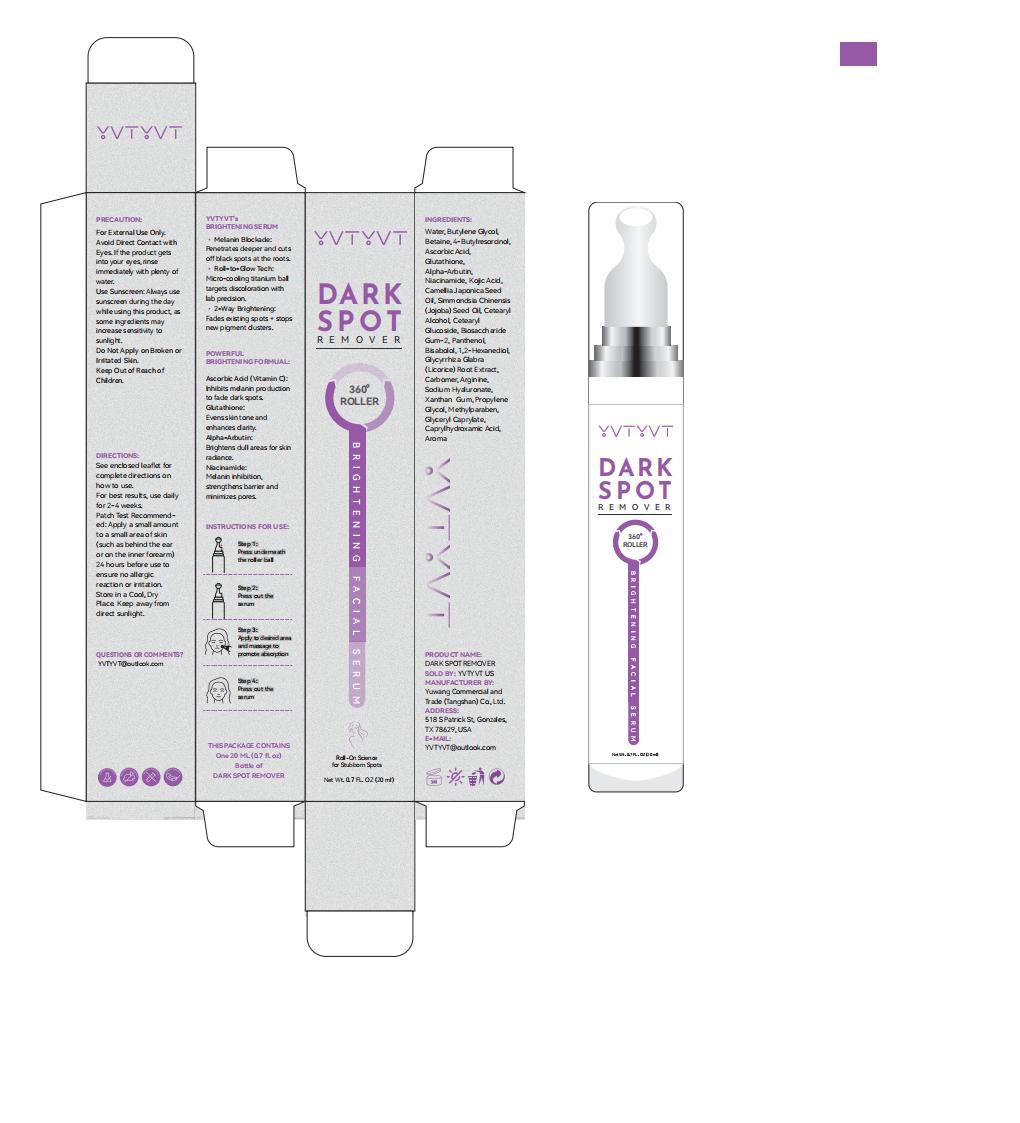
INDICATIONS & USAGE SECTION
Used to remove dark spots
Reduce pigmentation
For skin moisturizing
STORAGE AND HANDLING SECTION
DOSAGE & ADMINISTRATION SECTION
For best results, use daily for 2-4 weeks
OTC - WHEN USING SECTION
Step 1: Cleanse skin to remove impurities.
Step 2: Apply appropriate amount post - cleansing.
Step 3: Use before heavy moisturizers or oils for better absorption.
OTC - STOP USE SECTION
If the product gets into your eyes, rinse immediately with plenty of water.
Discontinue Use if Irritation Occurs: If redness, itching, or irritation
develops, stop use and consult a dermatologist.
OTC - DO NOT USE SECTION
Do Not Apply on Broken or Irritated Skin.
OTC - ACTIVE INGREDIENT SECTION
ASCORBIC ACID, KOJIC ACID
ALARMS
For External Use Only.
Avoid Direct Contact with Eyes. If the product gets into your eyes, rinse
immediately with plenty of water.
Discontinue Use if Irritation Occurs: If redness, itching, or irritation
develops, stop use and consult a dermatologist.
Use Sunscreen: Always use sunscreen during the day while using this product,
as some ingredients may increase sensitivity to sunlight.
Do Not Apply on Broken or Irritated Skin.
Keep Out of Reach of Children.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
INACTIVE INGREDIENT SECTION
WATER
BUTYLENE GLYCOL
BETAINE
4-BUTYLRESORCINOL
GLUTATHIONE
NIACINAMIDE
CAMELLIA JAPONICA SEED OIL
SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL
CETEARYL ALCOHOL
CETEARYL GLUCOSIDE
BIOSACCHARIDE GUM-2
PANTHENOL
BISABOLOL
1,2-HEXANEDIOL
SODIUM HYALURONATE
XANTHAN GUM
PROPYLENE GLYCOL
METHYLPARABEN
GLYCERYL CAPRYLATE
WARNINGS SECTION
For External Use Only.
Avoid Direct Contact with Eyes.
If the product gets into your eyes, rinse immediately with plenty of water.
Discontinue Use if Irritation Occurs: If redness, itching, or irritation
develops, stop use and consult a dermatologist.
Do Not Apply on Broken or Irritated Skin. Keep Out of Reach of Children.
OTC - PURPOSE SECTION
Used to remove dark spots
