Ki No Sin
84540-323 Ki No Sin capsule
20cf8f80-c638-4d94-e063-6294a90a5400
HUMAN OTC DRUG LABEL
Aug 27, 2025
NPG Bio Pharmaceuticals Co.,Ltd.
DUNS: 963309698
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Calcium Carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Label
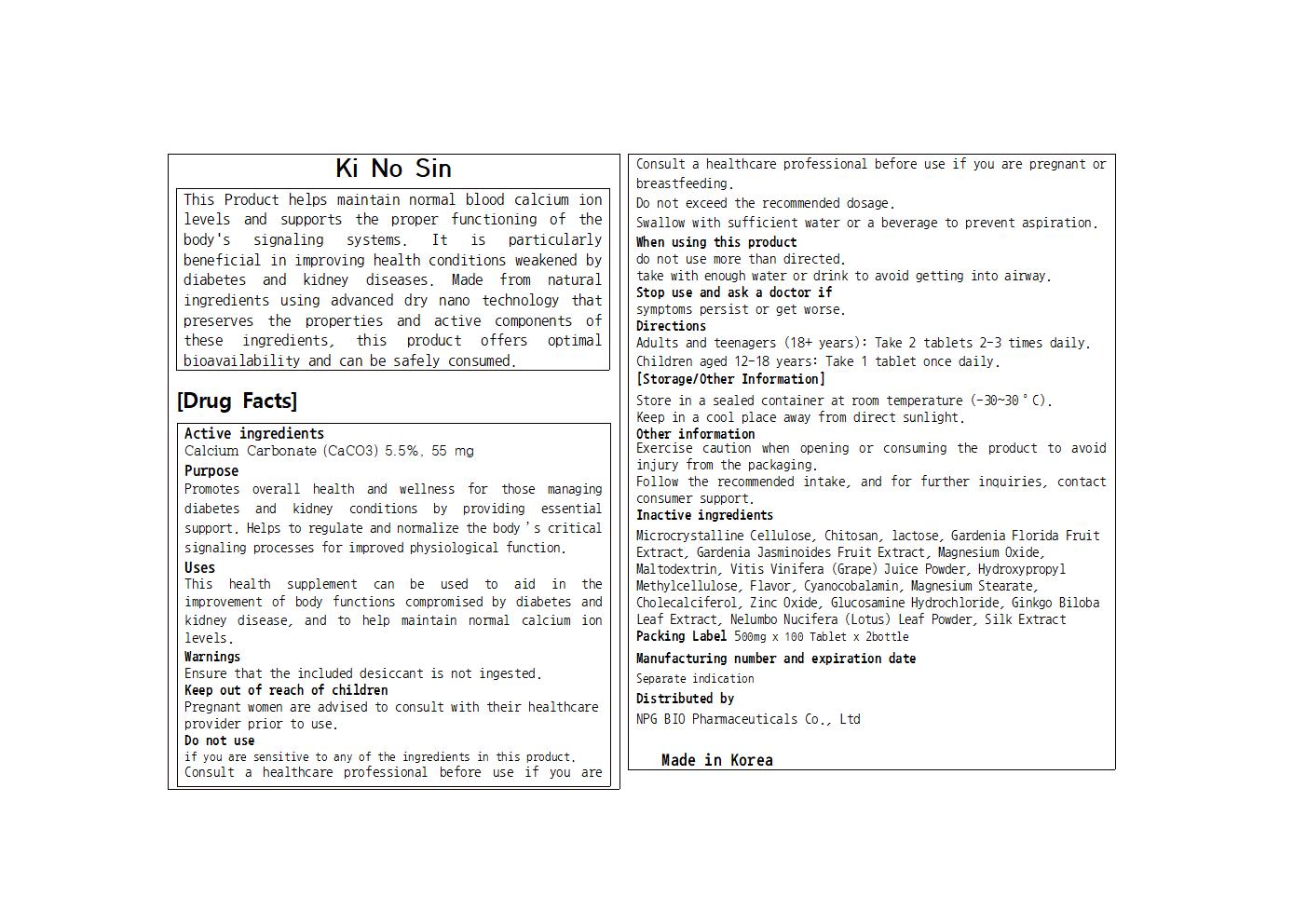
INDICATIONS & USAGE SECTION
Uses
This health supplement can be used to aid in the improvement of body functions compromised by diabetes and kidney disease, and to help maintain normal calcium ion levels.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients
Calcium Carbonate (CaCO3) 5.5%, 55 mg
OTC - PURPOSE SECTION
Purposes
Promotes overall health and wellness for those managing diabetes and kidney conditions by providing essential support. Helps to regulate and normalize the body’s critical signaling processes for improved physiological function.
OTC - DO NOT USE SECTION
Warnings
if you are sensitive to any of the ingredients in this product.
Consult a healthcare professional before use if you are pregnant or breastfeeding.
Do not exceed the recommended dosage.
Swallow with sufficient water or a beverage to prevent aspiration.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Warnings
Keep out of reach of children
Pregnant women are advised to consult with their healthcare provider prior to use.
OTC - STOP USE SECTION
Warnings
Stop use and ask a doctor if
symptoms persist or get worse.
OTC - WHEN USING SECTION
Warnings
When using this product
do not use more than directed.
take with enough water or drink to avoid getting into airway.
WARNINGS SECTION
Warnings
Ensure that the included desiccant is not ingested.
DOSAGE & ADMINISTRATION SECTION
Directions
Adults and teenagers (18+ years): Take 2 tablets 2-3 times daily.
Children aged 12-18 years: Take 1 tablet once daily.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Microcrystalline Cellulose, Chitosan, lactose, Gardenia Florida Fruit Extract, Gardenia Jasminoides Fruit Extract, Magnesium Oxide, Maltodextrin, Vitis Vinifera (Grape) Juice Powder, Hydroxypropyl Methylcellulose, Flavor, Cyanocobalamin, Magnesium Stearate, Cholecalciferol, Zinc Oxide, Glucosamine Hydrochloride, Ginkgo Biloba Leaf Extract, Nelumbo Nucifera (Lotus) Leaf Powder, Silk Extract
