Clobetasol Propionate
Clobetasol Propionate Gel, 0.05% Clobetasol Propionate Cream USP, 0.05% Clobetasol Propionate Ointment USP, 0.05%
4a761afb-a48a-4a5a-8700-ad93601a260f
HUMAN PRESCRIPTION DRUG LABEL
Aug 7, 2025
Sun Pharmaceutical Indutries, Inc.
DUNS: 146974886
Taro Pharmaceuticals U.S.A., Inc.
DUNS: 145186370
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Clobetasol Propionate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Clobetasol Propionate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (20)
Clobetasol Propionate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPLE DISPLAY PANEL- 30G TUBE CARTON (SUN)
 NDC
51672-1294-2
NDC
51672-1294-2
Clobetasol Propionate Gel 0.05%
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
In a controlled clinical trial with clobetasol propionate gel, the only reported adverse reaction that was considered to be drug related was a report of burning sensation (1.8% of treated patients). In controlled clinical trials, the most frequent adverse reactions reported for clobetasol propionate cream were burning and stinging sensation in 1% of treated patients. Less frequent adverse reactions were itching, skin atrophy, and cracking and fissuring of the skin. In controlled clinical trials, the most frequent adverse events reported for clobetasol propionate ointment were burning sensation, irritation, and itching in 0.5% of treated patients. Less frequent adverse reactions were stinging, cracking, erythema, folliculitis, numbness of fingers, skin atrophy, and telangiectasia.
Cushing's syndrome has been reported in infants and adults as a result of prolonged use of topical clobetasol propionate formulations. The following additional local adverse reactions have been reported with topical corticosteroids, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions are listed in an approximately decreasing order of occurrence: dryness, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, irritation, striae, and miliaria.
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
SPL UNCLASSIFIED SECTION
Mfd. by: Sun Pharma Canada Inc., Brampton, Ontario, Canada L6T 1C1
Dist by:**Sun Pharmaceutical Industries,**Inc., Cranbury, NJ 08512
Revised: July 2025
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Like other topical corticosteroids, clobetasol propionate has anti- inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A 2inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressings with hydrocortisone for up to 24 hours has not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption. Greater absorption was observed for the clobetasol propionate gel formulation as compared to the cream formulation in in vitro human skin penetration studies. Studies performed with clobetasol propionate gel, cream and ointment indicate that they are in the super-high range of potency as compared with other topical corticosteroids.
HOW SUPPLIED SECTION
HOW SUPPLIED
Clobetasol Propionate Gel, 0.05% is supplied in tamper-evident tubes:15 g (NDC 51672-1294-1), 30 g (NDC 51672-1294-2), and 60 g (NDC 51672-1294-3).
Clobetasol Propionate Cream USP, 0.05% is supplied in tamper-evident tubes: 15 g (NDC 51672-1258-1), 30 g (NDC 51672-1258-2), 45 g (NDC 51672-1258-6), and 60 g (NDC 51672-1258-3).
Clobetasol Propionate Ointment USP, 0.05% is supplied in tamper-evident tubes: 15 g (NDC 51672-1259-1), 30 g (NDC 51672-1259-2), 45 g (NDC 51672-1259-6), and 60 g (NDC 51672-1259-3).
Store at 20°-25°C (68°-77°F)[see USP Controlled Room Temperature].DO NOT REFRIGERATE
DESCRIPTION SECTION
DESCRIPTION
Clobetasol propionate gel, cream and ointment contain the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity.
Clobetasol propionate is a white to cream-colored crystalline powder insoluble in water. Chemically, it is 21-chloro-9-fluoro-11β,17-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17-propionate, and it has the following structural formula:
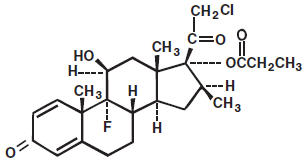
Each gram of the 0.05% gel contains 0.5 mg clobetasol propionate in a base of carbomer 934P, propylene glycol, purified water, and sodium hydroxide.
Each gram of the 0.05% cream contains clobetasol propionate 0.5 mg in a cream base of cetyl alcohol, chlorocresol, citric acid, glyceryl monostearate, glyceryl stearate/polyethylene glycol 100 stearate, propylene glycol, purified water, sodium citrate, stearyl alcohol, and white wax.
Each gram of the 0.05% ointment contains clobetasol propionate 0.5 mg in a base of propylene glycol, sorbitan sesquioleate, and white petrolatum.
