Medi-First Burn
Medi-First Burn Gel Drug Facts
44bb5f25-1114-4bb1-8bcf-40402594abc4
HUMAN OTC DRUG LABEL
Sep 9, 2025
Unifirst First Aid Corporation
DUNS: 832947092
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lidocaine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
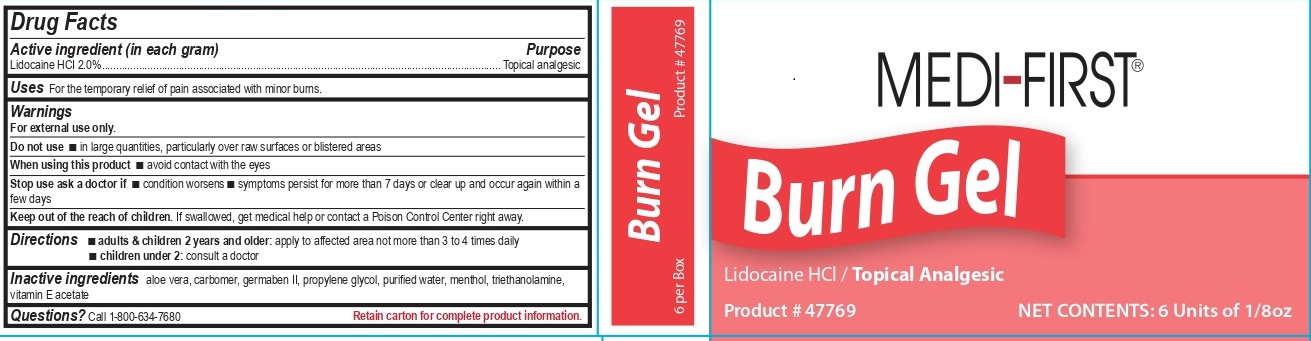
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Medi-First Burn Gel Label
Medi-First ®
Burn Gel
Lidocaine HCl / Topical Analgesic
Product # 47769
NET CONTENTS: 6 Units of 1/8 oz
INDICATIONS & USAGE SECTION
Uses
For the temporary relief of pain associated with minor burns.
SPL UNCLASSIFIED SECTION
Drug Facts
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each gram)
Lidocaine HCI 2.0%
OTC - PURPOSE SECTION
Purpose
Topical analgesic
WARNINGS SECTION
Warnings
For external use only.
Do not use
- in large quantities, particularly over raw or blistered areas
When using this product
- avoid contact with the eyes
Stop use ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clears up and occurs again within a few days
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
*adults & children 2 years and older: apply to affected area not more than 3 to 4 times daily *children under 2: consult a doctor
INACTIVE INGREDIENT SECTION
Inactive ingredients
aloe vera, carbomer, germaben II, propylene glycol, purified water, menthol, triethanolamine, vitamin E acetate
OTC - QUESTIONS SECTION
Questions?
Call 1-800-634-7680
Mfg. for Medique Products, 17080 Alico Commerce Ct., Ste 1, Fort Myers, FL 33967
