Plegridy
These highlights do not include all the information needed to use PLEGRIDY safely and effectively. See full prescribing information for PLEGRIDY. PLEGRIDY (peginterferon beta-1a) injection, for subcutaneous or intramuscular useInitial U.S. Approval: 2014
08f0ea03-4e6d-195d-aef4-886e32befa95
HUMAN PRESCRIPTION DRUG LABEL
Jul 31, 2023
Biogen Inc.
DUNS: 121376230
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
peginterferon beta-1a
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
peginterferon beta-1a
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
peginterferon beta-1a
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
peginterferon beta-1a
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
peginterferon beta-1a
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel –Carton Label
NDC 64406-017-01
Plegridy**®******
****(peginterferon beta-1a)
Injection
125 mcg/0.5 mL
For intramuscular use only
Every 14 Days
Contents:
2 Single-Dose Prefilled Syringes and
2 Needles for Injection
Every 14 Days
Dispense with enclosed
Medication Guide
Rx only

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hepatic Injury
Severe hepatic injury, including hepatitis, autoimmune hepatitis, and rare cases of severe hepatic failure, have been reported with interferon beta. Asymptomatic elevation of hepatic transaminases has also been reported, and in some patients has recurred upon rechallenge with interferon beta.
Elevations in hepatic enzymes and hepatic injury have been observed with the use of PLEGRIDY in clinical studies. The incidence of increases in hepatic transaminases was greater in patients taking PLEGRIDY than in those taking placebo. The incidence of elevations of alanine aminotransferase above 5 times the upper limit of normal was 1% in placebo-treated patients and 2% in PLEGRIDY-treated patients. The incidence of elevations of aspartate aminotransferase above 5 times the upper limit of normal was less than 1% in placebo-treated patients and less than 1% in PLEGRIDY-treated patients. Elevations of serum hepatic transaminases combined with elevated bilirubin occurred in 2 patients. Both cases resolved following discontinuation of PLEGRIDY.
Cases of noninfectious hepatitis have been reported in the postmarketing setting with use of PLEGRIDY.
Monitor patients for signs and symptoms of hepatic injury.
5.2 Depression and Suicide
Depression, suicidal ideation, and suicide occur more frequently in patients receiving interferon beta than in patients receiving placebo.
In clinical studies, the overall incidence of adverse events related to depression and suicidal ideation in multiple sclerosis patients was 8% in both the PLEGRIDY and placebo groups. The incidence of serious events related to depression and suicidal ideation was similar and less than 1% in both groups.
Advise patients to report immediately any symptom of depression or suicidal ideation to their healthcare provider. If a patient develops depression or other severe psychiatric symptoms, consider stopping treatment with PLEGRIDY.
5.3 Anaphylaxis and Other Allergic Reactions
Serious allergic reactions are rare complications of treatment with interferon beta; anaphylaxis has been reported with use of PLEGRIDY in the postmarketing setting.
Less than 1% of PLEGRIDY-treated patients experienced a serious allergic reaction such as angioedema or urticaria. Those who did have serious allergic reactions recovered promptly after treatment with antihistamines or corticosteroids. Discontinue PLEGRIDY if a serious allergic reaction occurs.
The protective rubber cover of the PLEGRIDY prefilled syringe for intramuscular administration contains natural rubber latex which may cause allergic reactions and should not be handled by latex-sensitive individuals. The safe use of PLEGRIDY prefilled syringe in latex-sensitive individuals has not been studied.
5.4 Injection Site Reactions Including Necrosis
Injection site reactions, including injection site necrosis, can occur with the use of interferon beta, including PLEGRIDY.
In clinical studies of subcutaneous PLEGRIDY, the incidence of injection site reactions (e.g., injection site erythema, pain, pruritus, or edema) was 66% in the PLEGRIDY group and 11% in the placebo group; the incidence of severe injection site reactions was 3% in the PLEGRIDY group and 0% in the placebo group. One patient out of 1468 patients who received PLEGRIDY in clinical studies experienced injection site necrosis. The injury resolved with standard medical treatment.
In Study 3, which compared single doses of intramuscular and subcutaneous PLEGRIDY [see Adverse Reactions (6.1)], the incidence of injection site reactions (e.g., injection site erythema, pain, pruritus, or edema) was 14% in the intramuscular PLEGRIDY group and 32% in the subcutaneous PLEGRIDY group.
Injection site abscesses and cellulitis have been reported in the postmarketing setting with use of interferon beta. Some cases required treatment with hospitalization for surgical drainage and intravenous antibiotics.
Periodically evaluate patient understanding and use of aseptic self-injection techniques and procedures, particularly if injection site necrosis has occurred.
Decisions to discontinue therapy following necrosis at a single injection site should be based on the extent of the necrosis. For patients who continue therapy with PLEGRIDY after injection site necrosis has occurred, avoid administration of PLEGRIDY near the affected area until it is fully healed. If multiple lesions occur, change injection site or discontinue PLEGRIDY until healing occurs.
5.5 Congestive Heart Failure
Congestive heart failure, cardiomyopathy, and cardiomyopathy with congestive heart failure occur in patients receiving interferon beta.
In clinical studies, the incidence of cardiovascular events was 7% in both PLEGRIDY and placebo treatment groups. No serious cardiovascular events were reported in the PLEGRIDY group.
Monitor patients with significant cardiac disease for worsening of their cardiac condition during initiation and continuation of treatment with PLEGRIDY.
5.6 Decreased Peripheral Blood Counts
Interferon beta can cause decreased peripheral blood counts in all cell lines, including rare instances of pancytopenia and severe thrombocytopenia.
In clinical studies, decreases in white blood cell counts below 3.0 x 109/L occurred in 7% of patients receiving PLEGRIDY and in 1% receiving placebo. There is no apparent association between decreases in white blood cell counts and an increased risk of infections or serious infections. The incidence of clinically significant decreases in lymphocyte counts (below 0.5 x 109/L), neutrophil counts (below 1.0 x 109/L), and platelet counts (below 100 x 109/L) were all less than 1% and similar in both placebo and PLEGRIDY groups. Two serious cases were reported in patients treated with PLEGRIDY: one patient (less than 1%) experienced severe thrombocytopenia (defined as a platelet count less than or equal to 10 x 109/L), and another patient (less than 1%) experienced severe neutropenia (defined as a neutrophil count less than or equal to 0.5 x 109/L). In both patients, cell counts recovered after discontinuation of PLEGRIDY. Compared to placebo, there were no significant differences in red blood cell counts in patients treated with PLEGRIDY.
Monitor patients for infections, bleeding, and symptoms of anemia. Monitor complete blood cell counts, differential white blood cell counts, and platelet counts during treatment with PLEGRIDY. Patients with myelosuppression may require more intensive monitoring of blood cell counts.
5.7 Thrombotic Microangiopathy
Cases of thrombotic microangiopathy (TMA), including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome, some fatal, have been reported with interferon beta products. Cases have been reported several weeks to years after starting interferon beta products. Discontinue PLEGRIDY if clinical symptoms and laboratory findings consistent with TMA occur, and manage as clinically indicated.
5.8 Pulmonary Arterial Hypertension
Cases of pulmonary arterial hypertension (PAH) have been reported in patients treated with interferon beta products, including PLEGRIDY. PAH has occurred in patients treated with interferon beta products in the absence of other contributory factors. Many of the reported cases required hospitalization, including one case with interferon beta in which the patient underwent a lung transplant. PAH has developed at various time points after initiating therapy with interferon beta products and may occur several years after starting treatment.
Patients who develop unexplained symptoms (e.g., dyspnea, new or increasing fatigue) should be assessed for PAH. If alternative etiologies have been ruled out and a diagnosis of PAH is confirmed, discontinue treatment and manage as clinically indicated.
5.9 Autoimmune Disorders
Autoimmune disorders of multiple target organs including idiopathic thrombocytopenia, hyper- and hypothyroidism, and autoimmune hepatitis have been reported with interferon beta.
In clinical studies, the incidence of autoimmune disorders was less than 1% in both PLEGRIDY and placebo treatment groups.
If patients develop a new autoimmune disorder, consider stopping PLEGRIDY.
5.10 Seizures
Seizures are associated with the use of interferon beta.
The incidence of seizures in multiple sclerosis clinical studies was less than 1% in patients receiving PLEGRIDY and placebo.
Exercise caution when administering PLEGRIDY to patients with a seizure disorder.
- Hepatic injury: monitor liver function tests; monitor patients for signs and symptoms of hepatic injury; consider discontinuation of PLEGRIDY if hepatic injury occurs (5.1)
- Depression and suicide: advise patients to report immediately any symptom of depression or suicidal ideation to their healthcare provider; consider discontinuation of PLEGRIDY if depression occurs (5.2)
- Anaphylaxis and other allergic reactions: Discontinue PLEGRIDY if a serious allergic reaction occurs (5.3)
- Injection site reactions: Do not administer PLEGRIDY into affected area until fully healed; if multiple lesions occur, change injection site or discontinue PLEGRIDY until healing of skin lesions (5.4)
- Congestive heart failure: monitor patients with pre-existing significant cardiac disease for worsening of cardiac symptoms (5.5)
- Decreased peripheral blood counts: monitor complete blood counts (5.6)
- Thrombotic Microangiopathy: Cases of thrombotic microangiopathy have been reported with interferon beta products. Discontinue PLEGRIDY if clinical symptoms and laboratory findings consistent with TMA occur (5.7)
- Pulmonary Arterial Hypertension: Cases of pulmonary arterial hypertension (PAH) have been reported in patients treated with interferon beta products, including PLEGRIDY. Discontinue PLEGRIDY if PAH is diagnosed (5.8)
- Autoimmune disorders: consider discontinuation of PLEGRIDY if a new autoimmune disorder occurs (5.9)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
PLEGRIDY is a clear to slightly opalescent and colorless to slightly yellow solution.
Subcutaneous Administration:
- Injection: 125 mcg/0.5 mL in a single-dose prefilled pen or single-dose prefilled syringe
- Injection: 63 mcg/0.5 mL in a single-dose prefilled pen or a single-dose prefilled syringe
- Injection: 94 mcg/0.5 mL in a single-dose prefilled pen or a single-dose prefilled syringe
Intramuscular Administration:
- Injection: 125 mcg/0.5 mL in a single-dose prefilled syringe
Subcutaneous Administration:
- Injection: 125 mcg/0.5 mL in a single-dose prefilled pen or single-dose prefilled syringe (3)
- Injection: 63 mcg/0.5 mL in a single-dose prefilled pen or single-dose prefilled syringe (3)
- Injection: 94 mcg/0.5 mL in a single-dose prefilled pen or single-dose prefilled syringe (3)
Intramuscular Administration:
- Injection: 125 mcg/0.5 mL solution in a single-dose prefilled syringe (3)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism by which PLEGRIDY exerts its effects in patients with multiple sclerosis is unknown.
12.2 Pharmacodynamics
There is no biochemical or physiologic effect known to relate directly to the clinical effect of PLEGRIDY.
12.3 Pharmacokinetics
After single-dose or multiple-dose subcutaneous administration of PLEGRIDY to healthy subjects, serum PLEGRIDY peak concentration (Cmax) and total exposure over time (area under the curve, or AUC) increased in proportion to doses from 63 to 188 micrograms. PLEGRIDY did not accumulate in the serum after multiple doses of 125 micrograms every 14 days. Pharmacokinetic parameters for PLEGRIDY, including Cmax and AUC, did not differ significantly between healthy volunteers and multiple sclerosis patients or between single-dose and multiple-dose administrations. However, the coefficient of variation between individual patients for AUC, Cmax, and half-life was high (41% to 68%, 74% to 89%, and 45% to 93%, respectively).
Absorption
After 125 microgram subcutaneous doses of PLEGRIDY in multiple sclerosis patients, the maximum concentration occurred between 1 and 1.5 days, the mean Cmax was 280 pg/mL, and the AUC over the 14 day dosing interval was 34.8 ng.hr/mL.
Distribution
In multiple sclerosis patients taking 125 microgram subcutaneous doses of PLEGRIDY every 14 days, the estimated volume of distribution was 481 liters.
Metabolism and Elimination
Clearance mechanisms for PLEGRIDY include catabolism and excretion. The major pathway of elimination is renal. The half-life is approximately 78 hours in multiple sclerosis patients. The mean steady state clearance of PLEGRIDY is approximately 4.1 L/hr. PLEGRIDY is not extensively metabolized in the liver.
The pharmacokinetics of 125 μg single dose of PLEGRIDY administered subcutaneously and intramuscularly were similar.
Specific Populations
Body weight, gender, and age do not require dosage adjustment.
Renal impairment can increase the Cmax and AUC for PLEGRIDY. Results of a pharmacokinetic study in patients with mild, moderate, and severe renal impairment (creatinine clearance 50 to 80, 30 to 50, and less than 30 mL/minute, respectively) showed increases above normal for Cmax of 27%, 26%, and 42%, and for AUC, increases of 30%, 40%, and 53%. The half-life was 53, 49, and 82 hours in patients with mild, moderate, and severe renal impairment, respectively, compared to 54 hours in normal subjects.
In the same study, subjects with end stage renal disease requiring hemodialysis two or three times weekly had AUC and Cmax of PLEGRIDY values that were similar to those of normal controls. Each hemodialysis session removed approximately 24% of circulating PLEGRIDY from the systemic circulation [see Use in Specific Populations (8.6)].
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
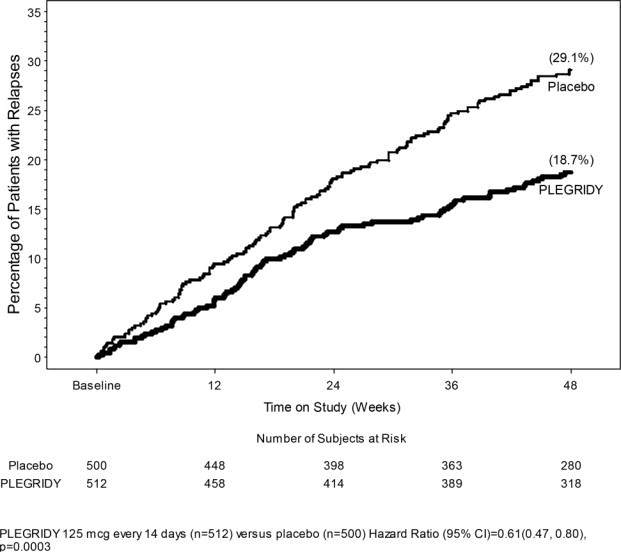
The efficacy of PLEGRIDY was demonstrated in the randomized, double-blind, and placebo-controlled phase (year 1) of Study 1. The trial compared clinical and MRI outcomes at 48 weeks in patients who received PLEGRIDY 125 micrograms (n=512) or placebo (n=500) by the subcutaneous route, once every 14 days.
Study 1 enrolled patients who had a baseline Expanded Disability Status Scale (EDSS) score from 0 to 5, who had experienced at least 2 relapses within the previous three years, and had experienced at least 1 relapse in the previous year. The trial excluded patients with progressive forms of multiple sclerosis. The mean age of the study population was 37 years, the mean disease duration was 3.6 years, and the mean EDSS score at baseline was 2.46. The majority of the patients were women (71%).
The trial scheduled neurological evaluations at baseline, every 12 weeks, and at the time of a suspected relapse. Brain MRI evaluations were scheduled at baseline, week 24, and week 48.
The primary outcome was the annualized relapse rate over 1 year. Secondary outcomes included the proportion of patients relapsing, number of new or newly enlarging T2 hyperintense lesions, and time to confirmed disability progression. Confirmed disability progression was defined as follows: if the baseline EDSS score was 0, a sustained 12-week increase in EDSS score of 1.5 points was required; if the baseline EDSS score was greater than 0, a sustained 12-week increase in EDSS score of 1 point was required. Table 4 and Figure 1 show the results of Study 1.
Table 4: Clinical and MRI Results of Study 1|
Endpoint |
PLEGRIDY |
Placebo |
p-value |
|---|---|---|---|
|
Clinical outcomes at 48 weeks |
N=512 |
N=500 | |
|
Annualized relapse rate |
0.26 |
0.40 |
0.0007 |
|
Relative reduction |
36% | ||
|
Proportion of patients with relapses |
0.19 |
0.29 |
0.0003 |
|
Relative risk reduction |
39% | ||
|
Proportion of patients with disability progression |
0.07 |
0.11 |
0.0383 |
|
Relative risk reduction |
38% | ||
|
MRI outcomes at 48 weeks |
N=457 |
N=476 | |
|
Mean number of new or newly enlarging T2 hyperintense lesions |
3.6 |
10.9 |
<0.0001 |
|
Relative reduction |
67% | ||
|
Mean number of Gd enhancing lesions |
0.2 |
1.4 |
<0.0001 |
|
Relative reduction |
86% |
Figure 1: Time to first relapse
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
PLEGRIDY (peginterferon beta-1a) injection is a sterile, preservative-free, clear to slightly opalescent and colorless to slightly yellow solution supplied as a 0.5 mL single-dose prefilled pen or a 0.5 mL single-dose prefilled syringe.
Subcutaneous Administration
PLEGRIDY (peginterferon beta-1a) injection for subcutaneous use is supplied as a single-dose prefilled pen or single-dose prefilled syringe with a rubber stopper and a 29-gauge, 0.5-inch staked needle with a rigid needle shield in the following packaging configurations:
- Carton containing two-125 mcg/0.5 mL single-dose prefilled pens of PLEGRIDY (NDC 64406-011-01).
- Starter Pack carton containing two single-dose prefilled pens; dose 1 provides 63 mcg/0.5 mL of PLEGRIDY and dose 2 provides 94 mcg/0.5 mL of PLEGRIDY (NDC 64406-012-01).
- Carton containing two-125 mcg/0.5 mL single-dose prefilled syringes of PLEGRIDY (NDC 64406-015-01).
- Starter Pack carton containing two single-dose prefilled syringes; dose 1 provides 63 mcg/0.5 mL of PLEGRIDY, and dose 2 provides 94 mcg/0.5 mL of PLEGRIDY (NDC 64406-016-01).
Intramuscular Administration
PLEGRIDY (peginterferon beta-1a) injection for intramuscular use is supplied as a single-dose prefilled syringe with a rubber stopper and a 23-gauge, 1.25-inch staked needle provided separately with the syringe in the following packaging configurations:
- Carton containing two-125 mcg/0.5 mL single-dose prefilled syringes of PLEGRIDY (NDC 64406-017-01).
- The PLEGRIDY Titration Kit must be prescribed and dispensed separately for treatment initiation. The Titration Kit contains two titration clips: The yellow clip (for dose 1) allows a delivered dose of 63 mcg of PLEGRIDY, and the purple clip (for dose 2) allows a delivered dose of 94 mcg of PLEGRIDY.
16.2 Storage and Handling
Store PLEGRIDY prefilled pens and prefilled syringes in a refrigerator between 2°C to 8°C (36°F to 46°F) in the closed original carton to protect from light until ready for injection. Do not freeze. Discard if frozen.
If refrigeration is unavailable, PLEGRIDY may be stored at room temperature up to 25°C (77°F) for a period up to 30 days, protected from light. PLEGRIDY can be removed from, and returned to, a refrigerator if necessary. The total combined time out of refrigeration should not exceed 30 days.
PLEGRIDY prefilled syringe for intramuscular administration contains natural rubber latex which may cause allergic reactions.
Dispose in a sharps-bin container or other hard plastic or metal sealable container. Always follow local regulations for disposal.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Instructions for Self-Injection Technique and Procedures
Provide appropriate instruction for methods of self-injection, including careful review of the PLEGRIDY Medication Guide and Instructions for Use. Instruct patients in the use of aseptic technique when administering PLEGRIDY.
Inform patients that a healthcare provider should show them or their caregiver how to prepare to inject PLEGRIDY before administering the first dose. Tell patients not to re-use needles or syringes, and instruct patients on safe disposal procedures. Inform patients to dispose of used needles and syringes in a puncture-resistant container, and instruct patients regarding safe disposal of full containers.
Advise patients:
- to rotate areas of injection with each dose to minimize the likelihood of injection site reactions [see Warnings and Precautions (5.4)]. For subcutaneous administration, the usual injection sites are the abdomen, back of the upper arm, and thigh. For intramuscular administration, alternate injections between the left and right thigh.
- NOT to inject into an area of the body where the skin is irritated, reddened, bruised, infected, or scarred in any way
- to check the injection site after 2 hours for redness, swelling, and tenderness
- to contact their healthcare professional if they have a skin reaction and it does not clear up in a few days
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant [see Use in Specific Populations (8.1)].
Liver Disease
Advise patients that severe hepatic injury, including rare cases of hepatic failure, has been reported during the use of interferon beta. Advise patients of symptoms of hepatic dysfunction, and instruct patients to report them immediately to their physician [see Warnings and Precautions (5.1)].
Depression and Suicide
Advise patients that depression, suicidal ideation, and suicide have been reported with the use of interferon beta. Instruct patients to report symptoms of depression or thoughts of suicide to their physician immediately [see Warnings and Precautions (5.2)].
Anaphylaxis and Other Allergic Reactions
Advise patients of the symptoms of allergic reactions and anaphylaxis, and instruct patients to seek immediate medical attention if these symptoms occur. Inform latex-sensitive patients that the PLEGRIDY prefilled syringe for intramuscular administration contains natural rubber latex [see Warnings and Precautions (5.3)].
Injection Site Reactions Including Necrosis
Advise patients that injection site reactions can occur and that the reactions can include injection site necrosis. Instruct patients to report promptly any break in the skin that is associated with blue-black discoloration, swelling, or drainage of fluid from the injection site [see Warnings and Precautions (5.4)].
Cardiac Disease
Advise patients that worsening of significant cardiac disease has been reported in patients using interferon beta. Advise patients of symptoms of worsening cardiac condition, and instruct patients to report them immediately to their physician [see Warnings and Precautions (5.5)].
Pulmonary Arterial Hypertension
Inform patients that PAH has occurred in patients treated with interferon beta products, including PLEGRIDY. Instruct patients to promptly report any new symptoms such as new or increasing fatigue or shortness of breath to their healthcare provider [see Warnings and Precautions (5.8)].
Seizure
Advise patients that seizures have been reported in patients using PLEGRIDY. Instruct patients to report seizures immediately to their physician [see Warnings and Precautions (5.10)].
Flu-like Symptoms
Inform patients that flu-like symptoms are common following initiation of therapy with PLEGRIDY. Prophylactic and concurrent use of analgesics and/or antipyretics may prevent or ameliorate flu-like symptoms sometimes experienced during interferon treatment [see Dosage and Administration (2.3) and Adverse Reactions (6.1)].
43643-09
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
U.S. License # 1697
1-800-456-2255
PLEGRIDY is a registered trademark of Biogen.
©2013-2023 Biogen
