LURBIPR
These highlights do not include all the information needed to use LURBIPR (FLURBIPROFEN TABLETS) safely and effectively. See full prescribing information for LURBIPR (FLURBIPROFEN TABLETS). FLURBIPROFEN tablets, for oral use Initial U.S. Approval: 1988
fe67c21f-0180-46af-9db0-ba9d40de3b6e
HUMAN PRESCRIPTION DRUG LABEL
Apr 28, 2025
New HeightsRx, LLC
DUNS: 119177168
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Flurbiprofen
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
NDC 84044-100-03
LURBIPR TM (Flurbiprofen
Tablets, USP)
100 mg
PHARMACIST: Dispense the
accompanying Medication
Guide to each patient.
Rx only
30 Tablets
New HeightsRx, LLC.

DESCRIPTION SECTION
11 DESCRIPTION
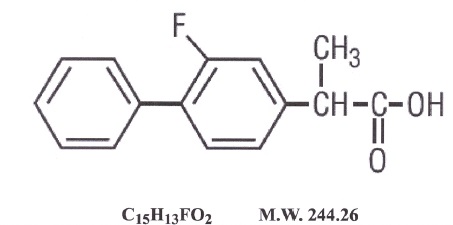 LURBIPR TM
(Flurbiprofen Tablets, USP) are a member of the phenylalkanoic acid derivative
group of nonsteroidal anti-inflammatory drug. LURBIPR TM (Flurbiprofen
Tablets, USP) are round, blue, film-coated debossed "NH" – "100" tablets for
oral administration. Flurbiprofen, USP is a racemic mixture of (+)S- and (-)R-
enantiomers. Flurbiprofen, USP is a white or slightly yellow crystalline
powder. It is slightly soluble in water at pH 7.0 and readily soluble in most
polar solvents. The chemical name is [1,1'-biphenyl]-4-acetic acid, 2-fluoro-
α-methyl-, (±)-. It has the following structural formula:
LURBIPR TM
(Flurbiprofen Tablets, USP) are a member of the phenylalkanoic acid derivative
group of nonsteroidal anti-inflammatory drug. LURBIPR TM (Flurbiprofen
Tablets, USP) are round, blue, film-coated debossed "NH" – "100" tablets for
oral administration. Flurbiprofen, USP is a racemic mixture of (+)S- and (-)R-
enantiomers. Flurbiprofen, USP is a white or slightly yellow crystalline
powder. It is slightly soluble in water at pH 7.0 and readily soluble in most
polar solvents. The chemical name is [1,1'-biphenyl]-4-acetic acid, 2-fluoro-
α-methyl-, (±)-. It has the following structural formula:
|
C 15H 13FO 2 M.W. 244.26 |
Each tablet, for oral administration, contains 100 mg flurbiprofen, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, titanium dioxide, and FD&C Blue #1 aluminum lake.
