Multivitamin with Fluoride
MULTIVITAMIN WITH FLUORIDE CHEWABLE TABLETS 0.25 mg, 0.5 mg and 1.0 mg Prescribing Information
a10ff759-eba7-414b-bb98-f17fbb6e6b57
HUMAN PRESCRIPTION DRUG LABEL
Aug 27, 2025
WINDER LABORATORIES, LLC
DUNS: 965195170
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Sodium Fluoride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Sodium Fluoride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
Sodium Fluoride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
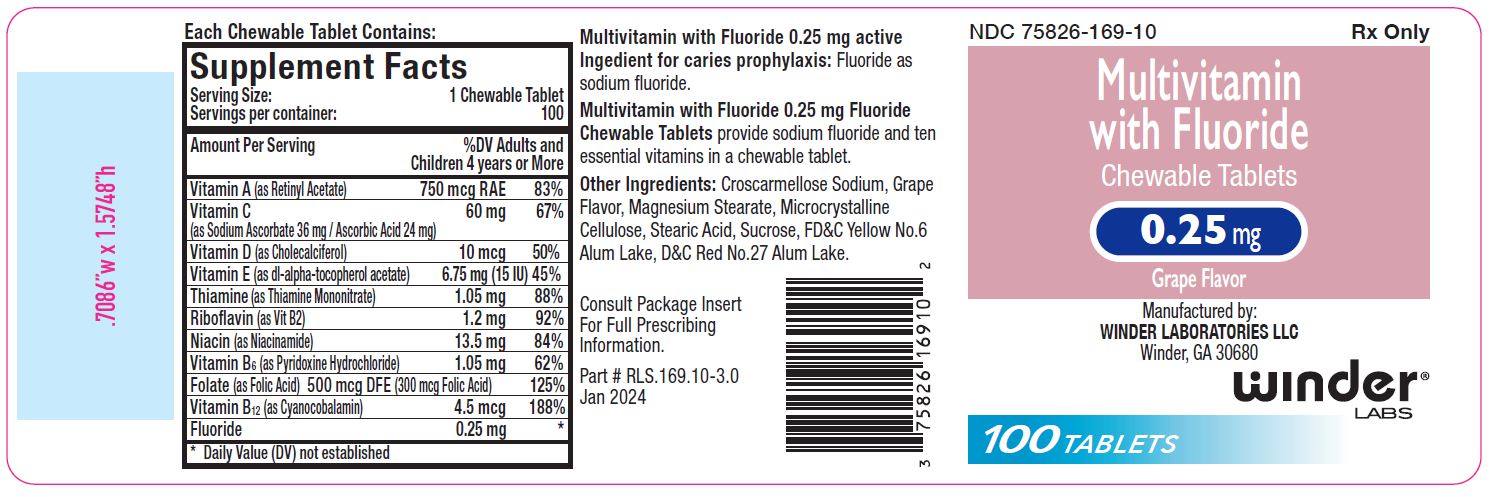
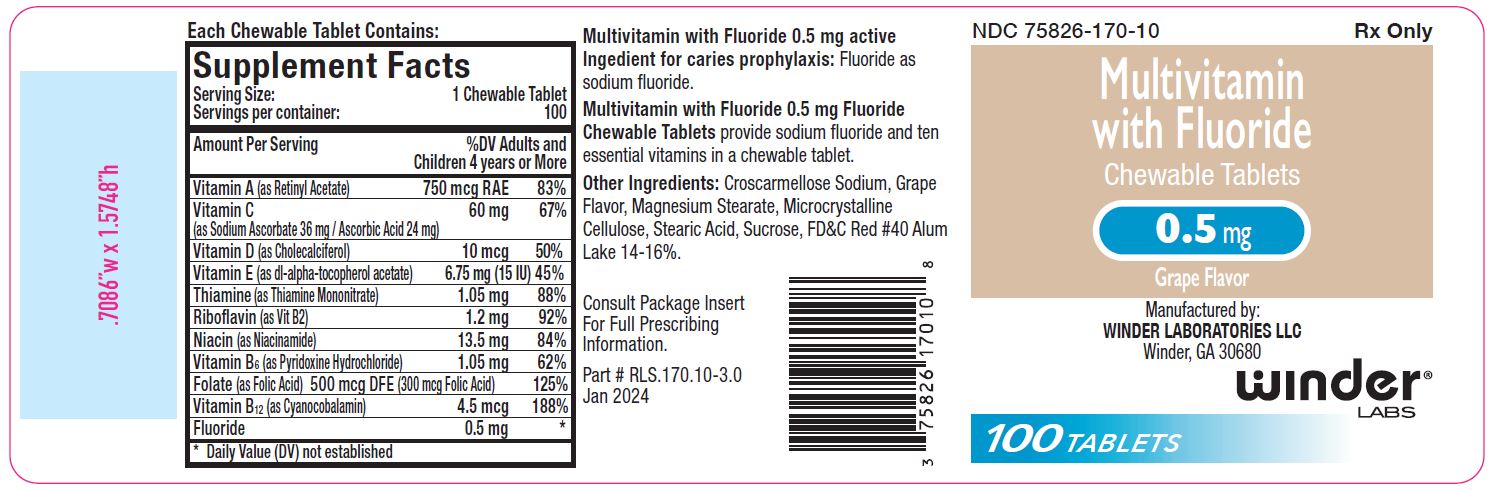
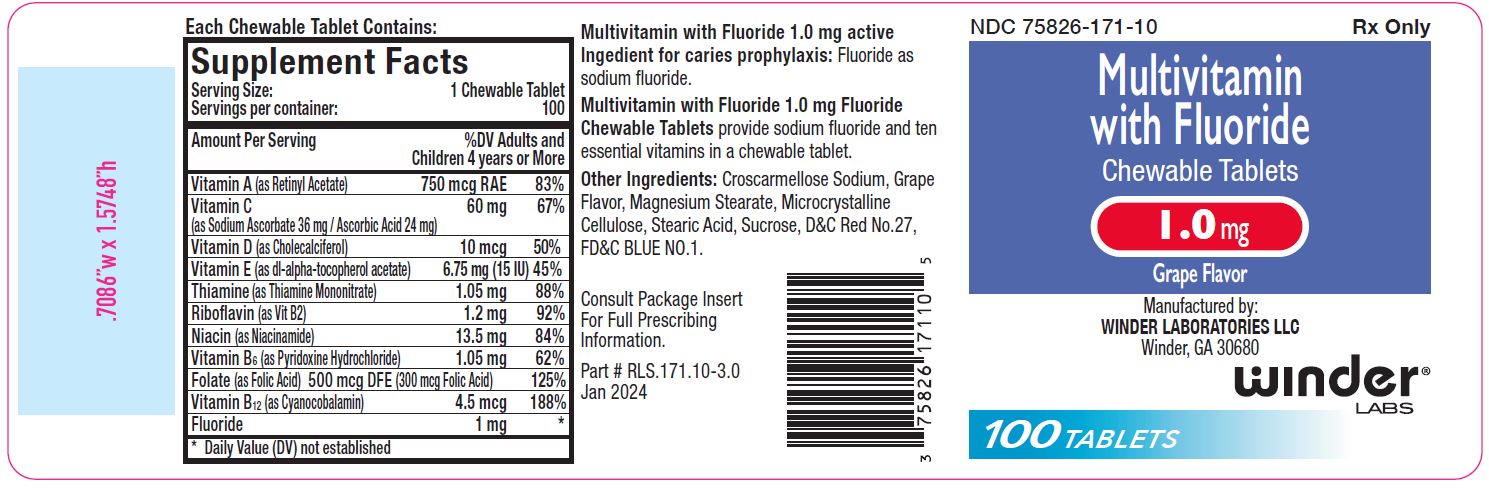
Supplementation of the diet with fluoride and ten essential vitamins.
Multivitamin with 1.0 mg Fluoride Chewable Tabletsprovide fluoride in tablet form for children 6-16 years of age in areas where the water fluoride level is less than 0.3 ppm.
Multivitamin with 0.5 mg Fluoride Chewable Tablets provide fluoride in tablet form for children 4-6 years of age where the water fluoride level is less than 0.3 ppm, and for children 6 years of age and above where the drinking water contains 0.3 through 0.6 ppm of fluoride.
Multivitamin with 0.25 mg Fluoride Chewable Tabletsprovide fluoride in tablet form for children 4-6 years of age where the drinking water contains 0.3 through 0.6 ppm of fluoride.
Multivitamin with Fluoride Chewable Tabletssupply significant amounts of
vitamins A, C, D, E, thiamine, riboflavin, niacin, vitamin B6, vitamin B12,
and folate to supplement the diet, and to help assure that nutritional
deficiencies of these vitamins will not develop.
Thus, in a single easy-to-use preparation, children obtain ten essential
vitamins and the important mineral, fluoride.
Supplementation of the diet with fluoride for caries prophylaxis.
The American Academy of Pediatrics recommends that children up to age 16, in
areas where drinking water contains less than optimal levels of fluoride,
receive daily fluoride supplementation.
Children usingMultivitamin with Fluoride Chewable Tabletsregularly should receive semiannual dental examinations. The regular brushing of teeth and attention to good oral hygiene practices are also essential.
Multivitamin with Fluoride Chewable tabletsare prescription product for the clinical dietary management of metabolic processes of caries prophylaxis and provides supplementation of the diet with ten essential vitamins.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Allergic rash and other idiosyncrasies have been rarely reported.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
It is well established that fluoridation of the water supply (1 ppm fluoride) during the period of tooth development leads to a significant decrease in the incidence of dental caries.
Multivitamin with Fluoride Chewable Tabletsprovide sodium fluoride and ten
essential vitamins in a chewable tablet. Because the tablets are chewable,
they provide a topical as well as systemic source of fluoride.
Hydroxyapatite is the principal crystal for all calcified tissue in the human
body. The fluoride ion reacts with the hydroxyapatite in the tooth as it is
formed to produce the more caries-resistant crystal, fluorapatite. The
reaction may be expressed by the equation:
Ca 10 (PO 4) 6(OH) 2+ 2F -------› Ca 10(PO 4)F 2+ 2OH -
(Hydroxyapatite) (Fluorapatite)
(Hydroxyapatite) (Fluorapatite)
Three stages of fluoride deposition in tooth enamel can be distinguished:
- Small amounts (reflecting the low levels of fluoride in tissue fluids) are incorporated into the enamel crystals while they are being formed.
- After enamel has been laid down, fluoride deposition continues in the surface enamel. Diffusion of fluoride from the surface inward is apparently restricted.
- After eruption, the surface enamel acquires fluoride from the water, food, supplementary fluoride and smaller amounts from saliva.
WARNINGS SECTION
WARNING
** Keep this and all medication out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.**
Keep this and all medication out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
CAUTION
****Should be chewed. This product, as with all chewable tablets, is not
recommended for children under age 4 due to risk of choking.
Do not eat or drink dairy products within one hour of fluoride administration.
PRECAUTIONS
The suggested dose ofMultivitamin with Fluoride Chewable Tabletsshould not be exceeded, since dental fluorosis may result from continued ingestion of large amounts of fluoride.
Before prescribingMultivitamin with Fluoride Chewable Tablets:
-
Determine the fluoride content of the drinking water from all major sources.
-
Make sure the child is not receiving significant amounts of fluoride from other sources such as medications and swallowed toothpaste.
-
Periodically check to make sure that the child does not develop significant dental fluorosis.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
One tablet daily or as prescribed.
HOW SUPPLIED SECTION
HOW SUPPLIED
3.25 mg: Multivitamin chewable tablets containing 0.25 mg fluoride are pink- colored, grape flavor, un-scored, round tablet, debossed with “WL” “169” on one side and plain on other side of tablet. Available in bottle of 100 (NDC 75826-169-10).
0.5 mg: Multivitamin chewable tablets containing 0.5 mg fluoride are peach- colored, grape flavor, un-scored, round tablet, debossed with “WL” “170” on one side and plain on other side of tablet. Available in bottle of 100 (NDC 75826-170-10).
1.0 mg: Multivitamin chewable tablets containing 1.0 mg fluoride are purple- colored, grape flavor, un-scored, round tablet, debossed with “WL” “171” on one side and plain on other side of tablet. Available in bottle of 100 (NDC 75826-171-10).
STORAGE
Store at 20° - 25°C (68°-77°F); excursions permitted to 15° - 30°C (59°-86°F).
See USP Controlled Room Temperature.
Dispense in a tight, light resistant container with a child-resistant closure as defined in the USP/NF. All prescription substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Manufactured by:
** Winder Laboratories LLC**
** Winder GA, 30680**
** RLS.170.99-3.0**
** Rev: 01/2024**
DESCRIPTION SECTION
Rx Only
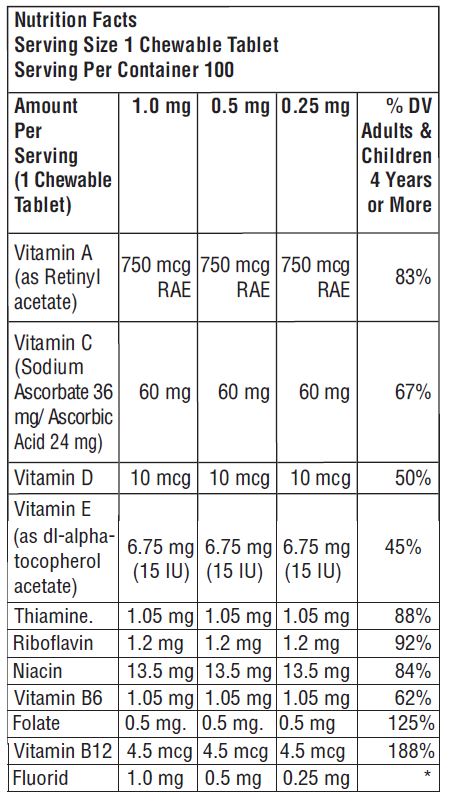
- Daily Value (% DV) not established
Active ingredients:
Fluoride as sodium fluoride.
Other Ingredients:
Croscarmellose Sodium, Grape Flavor, Magnesium Stearate, Microcrystalline
Cellulose, Stearic Acid, Sucrose.
0.25 mg also contains: D&C Red #27 Alum Lake and FD&C Yellow #6 Alum Lake.
0.5 mg also contains: FD&C Red #40 Alum Lake.
1.0 mg also contains: D&C Red #27 and FD&C Blue #1.
