Enoxaparin Sodium
ENOXAPARIN SODIUM injection, for subcutaneous and intravenous use
b4d9e4d4-4fdb-48d6-a169-7bc5eebc51e9
HUMAN PRESCRIPTION DRUG LABEL
Nov 5, 2022
Italfarmaco SpA
DUNS: 428179329
Products 7
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Enoxaparin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Enoxaparin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Enoxaparin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Enoxaparin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Enoxaparin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Enoxaparin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Enoxaparin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
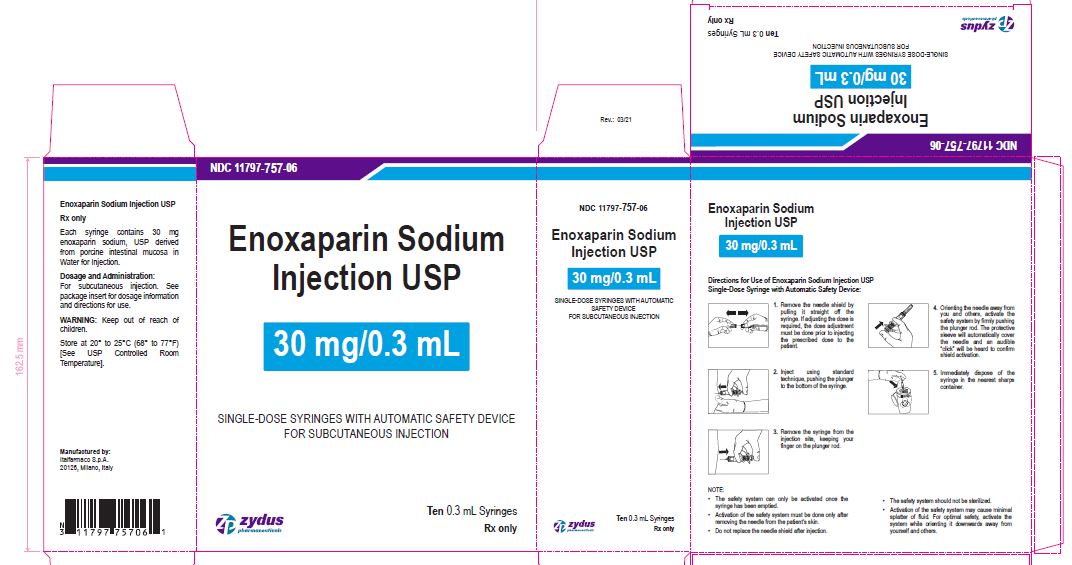
NDC 11797-757-06
Enoxaparin Sodium Injection USP
30 mg/0.3 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.3 mL Syringes
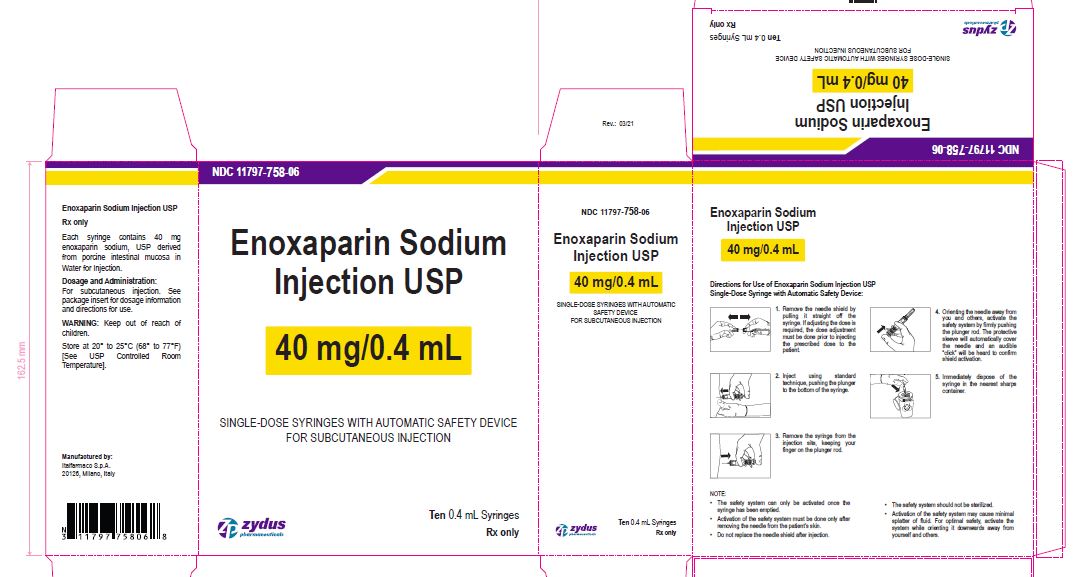
NDC 11797-758-06
Enoxaparin Sodium Injection USP
40 mg/0.4 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.4 mL Syringes
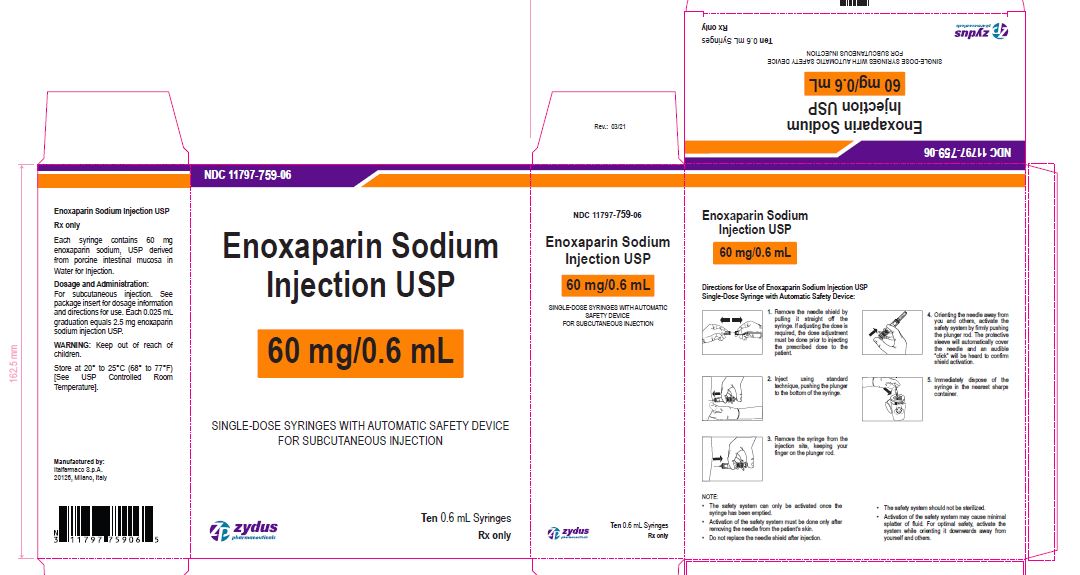
NDC11797-759-06
Enoxaparin Sodium Injection USP
60 mg/0.6 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.6 mL Syringes
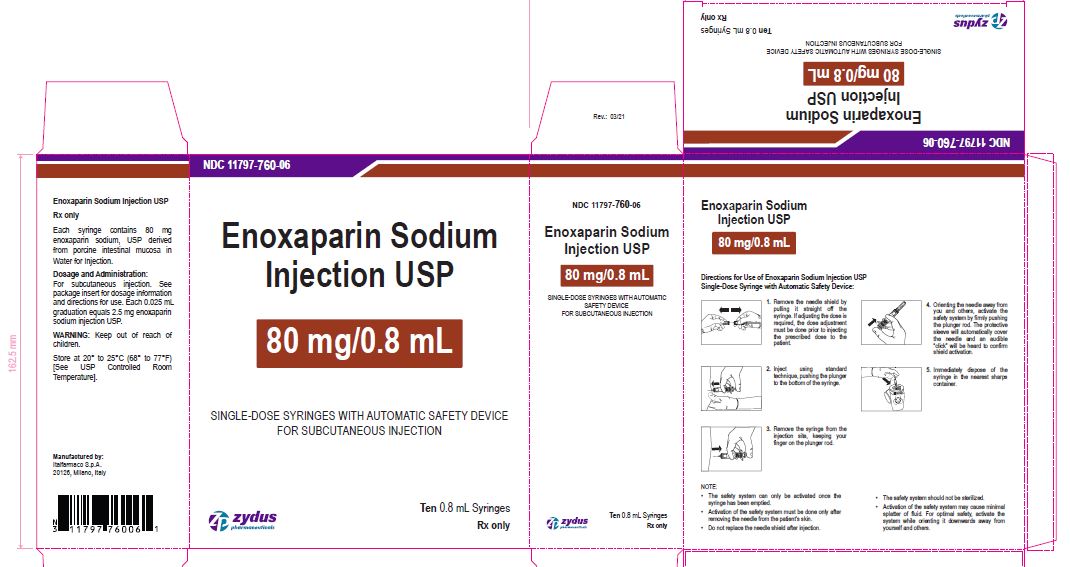
NDC11797-760-06
Enoxaparin Sodium Injection USP
80 mg/0.8 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8 mL Syringes
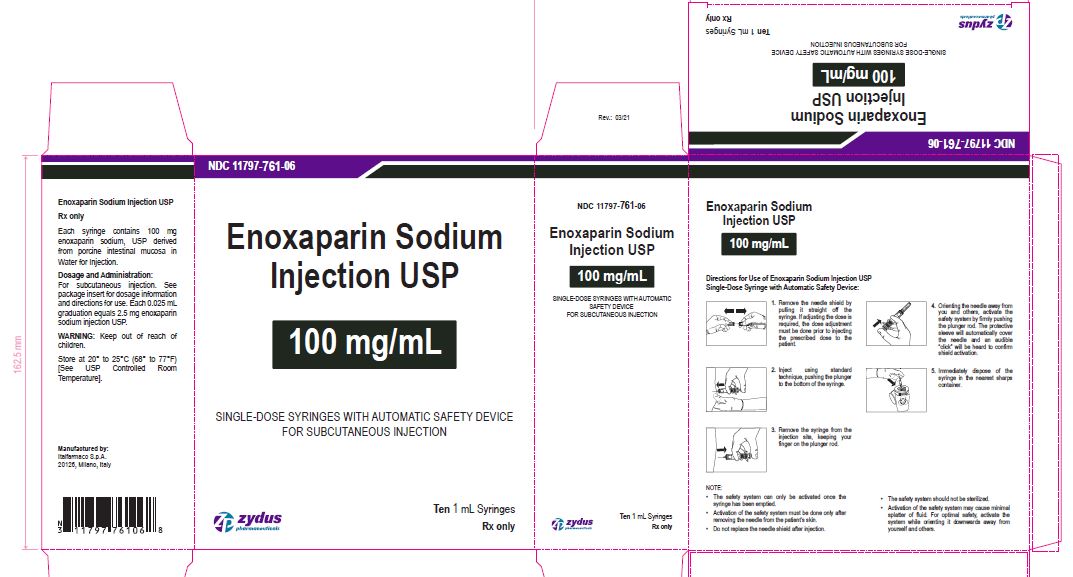
NDC 11797-761-06
Enoxaparin Sodium Injection USP
100 mg/1 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1 mL Syringes
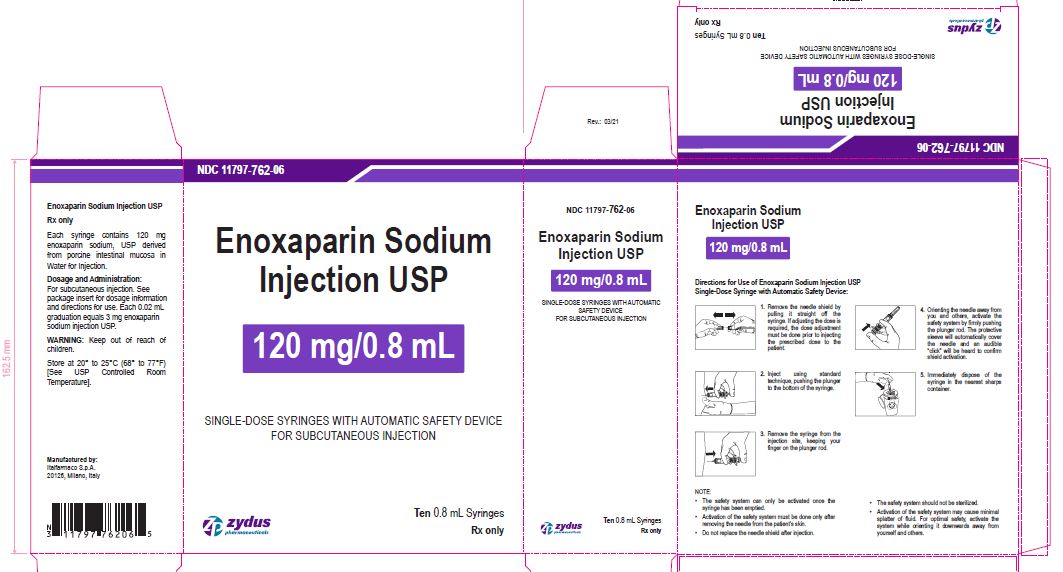
NDC11797-762-06
Enoxaparin Sodium Injection USP
120 mg/0.8 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8 mL Syringes
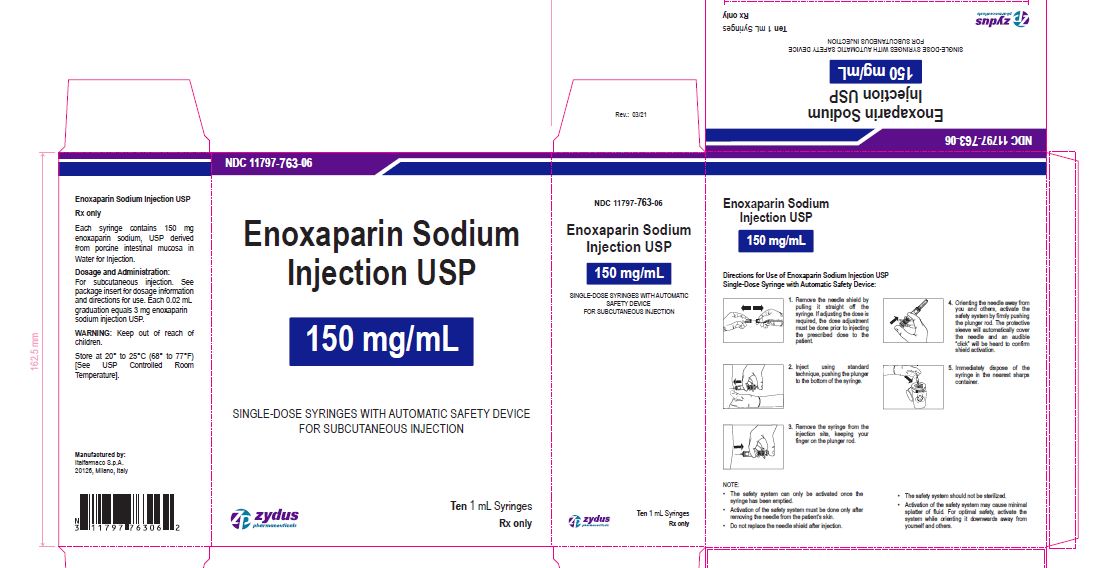
NDC****11797-763-06
Enoxaparin Sodium Injection USP
150 mg/1 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1 mL Syringes