Frindovyx
These highlights do not include all the information needed to use FRINDOVYX safely and effectively. See full prescribing information for FRINDOVYX. FRINDOVYX (cyclophosphamide) injection, for intravenous useInitial U.S. Approval: 1959
5ad8ea65-4c98-487a-9c8d-df778765ec5e
HUMAN PRESCRIPTION DRUG LABEL
Aug 11, 2025
Avyxa Pharma, LLC
DUNS: 128918748
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Cyclophosphamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Cyclophosphamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Cyclophosphamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
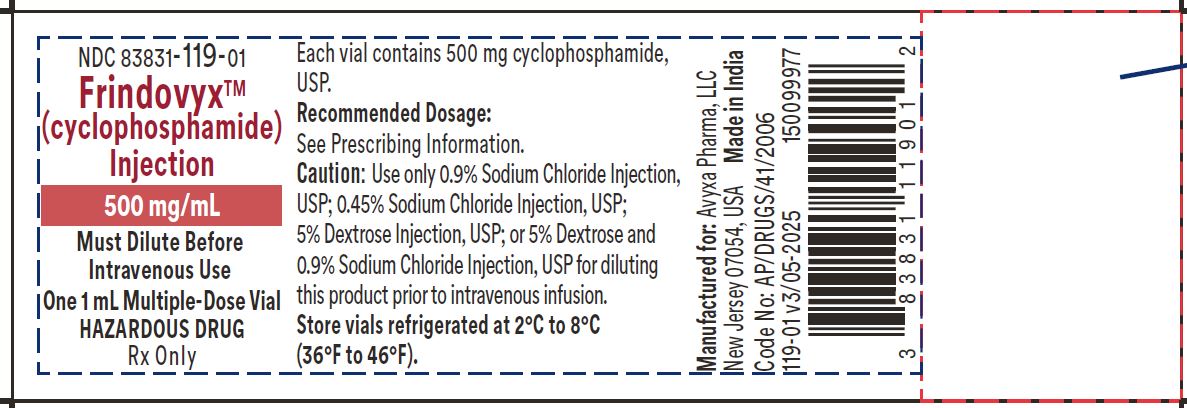
Frindovyx (cyclophosphamide) Injection, 500mg/mL - Vial Label
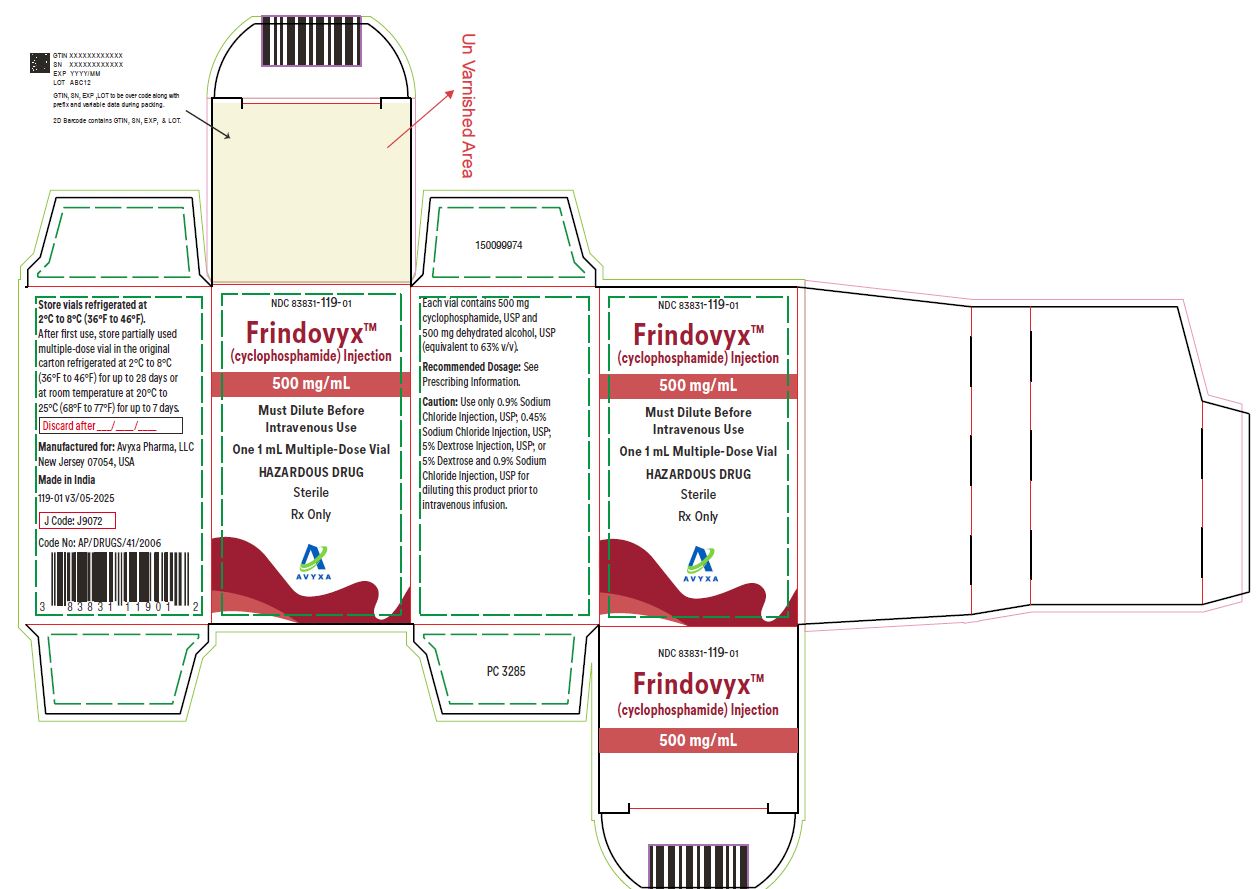
Frindovyx (cyclophosphamide) Injection, 500 mg/ mL - Carton Label
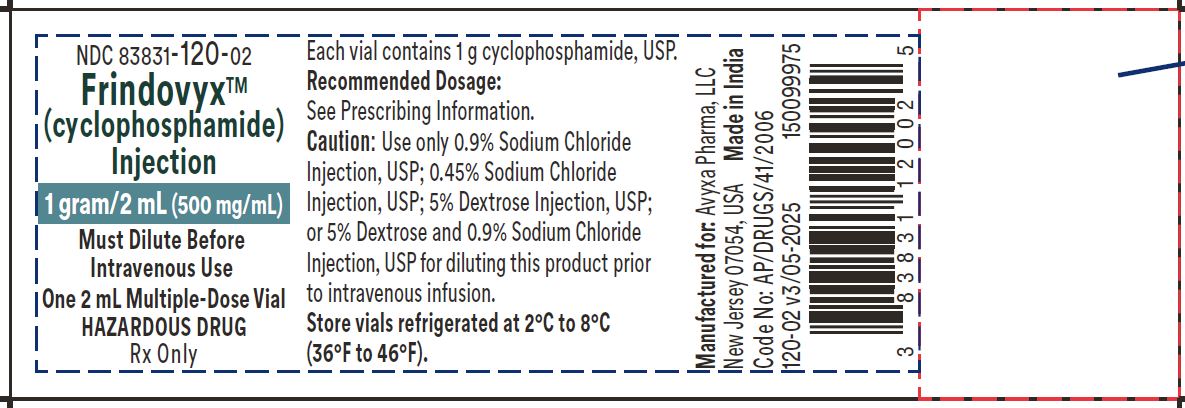
Frindovyx (cyclophosphamide) Injection, 1 g/2 mL - Vial Label
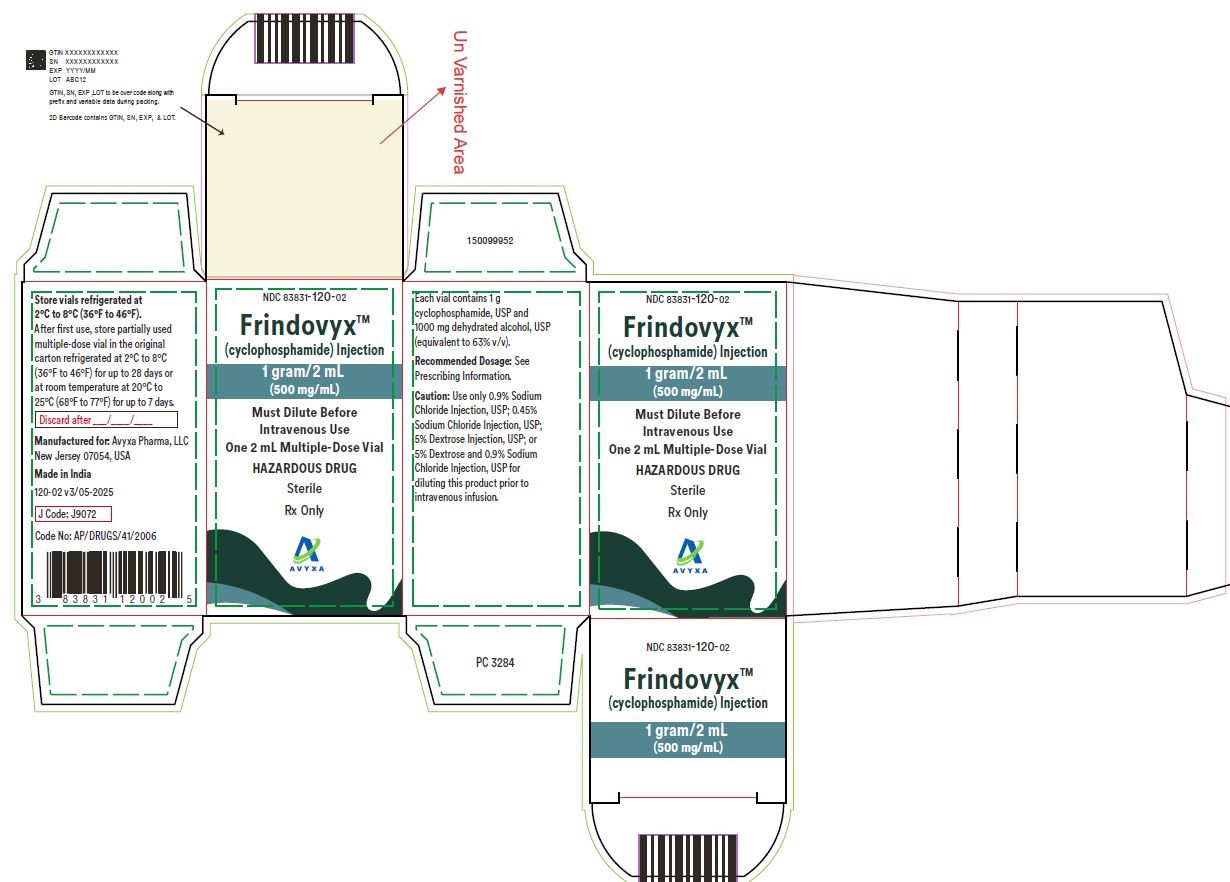

Frindovyx (cyclophosphamide) Injection, 2 g/4 mL - Vial Label
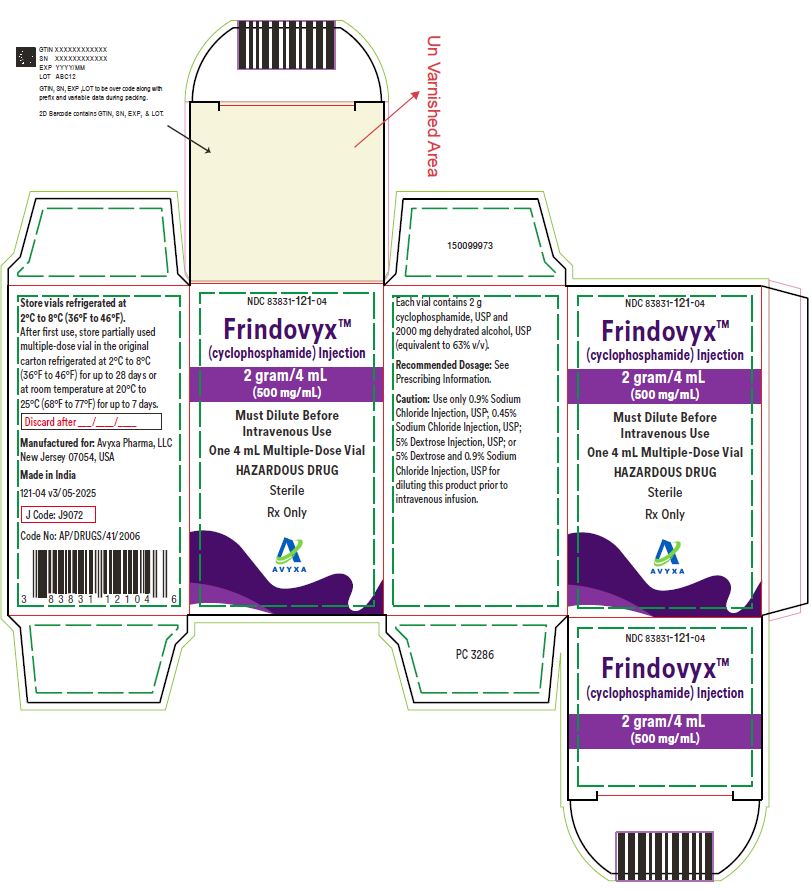
Frindovyx (cyclophosphamide) Injection, 2 g/ 4 mL - Carton Label
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Dosage and Administration (2.3) 7/2025
Dosage and Administration (2.3) 7/2025
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
During or immediately after the administration of FRINDOVYX, adequate amounts of fluid should be ingested or infused to force diuresis in order to reduce the risk of urinary tract toxicity. Therefore, FRINDOVYX should be administered in the morning.
2.2 Recommended Dosage for Malignant Diseases
Adults and Pediatric Patients
Intravenous
When used as the only oncolytic drug therapy, the recommended dosage for the initial course of FRINDOVYX for patients with no hematologic deficiency is 40 mg/kg to 50 mg/kg given intravenously in divided doses over a period of 2 to 5 days. Other intravenous regimens include 10 mg/kg to 15 mg/kg given every 7 to 10 days or 3 mg/kg to 5 mg/kg twice weekly.
Adjust the dosage of FRINDOVYX based on the specific regimen administered, response to treatment, myelosuppression or other adverse reactions, and patient risk factors [see Warnings and Precautions (5)].
2.3 Preparation, Handling and Administration
FRINDOVYX is a hazardous drug. Follow applicable special handling and disposal procedures1. Caution should be exercised when handling and preparing FRINDOVYX. To minimize the risk of dermal exposure, always wear gloves when handling vials containing FRINDOVYX.
FRINDOVYX
Intravenous Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use cyclophosphamide vials if there are signs of particulate matter.
Cyclophosphamide does not contain any antimicrobial preservative and thus care must be taken to assure the sterility of prepared solutions. Use aseptic technique.
For Direct Intravenous Injection
Withdraw the prescribed dose of FRINDOVYX from the vial with a syringe and dilute with 0.9% Sodium Chloride Injection, USP to a concentration of 20 mg/mL of cyclophosphamide.
For Intravenous Infusion
Withdraw the prescribed dose of FRINDOVYX from the vial with a syringe and dilute FRINDOVYX to a concentration of 2 mg/mL with any of the following diluents:
-
0.9% Sodium Chloride Injection, USP
-
0.45% Sodium Chloride Injection, USP
-
5% Dextrose Injection, USP
-
5% Dextrose and 0.9% Sodium Chloride Injection, USP
To reduce the likelihood of adverse reactions that appear to be administration rate-dependent (e.g., facial swelling, headache, nasal congestion, scalp burning), cyclophosphamide should be injected or infused very slowly. Duration of the infusion also should be appropriate for the volume and type of carrier fluid to be infused.
Storage of Diluted Cyclophosphamide Solution:
If not used immediately, for microbiological integrity, cyclophosphamide solutions should be stored as described in Table 1.
Table 1: Storage of Cyclophosphamide Solutions|
Diluent |
Storage | |
|
Room Temperature |
Refrigerated | |
|
** Diluted Solution (20 mg/mL) for Direct Intravenous Injection** | ||
|
0.9% Sodium Chloride Injection, USP |
up to 24 hours |
up to 6 days |
|
** Diluted Solutions (2 mg/mL) for Intravenous Infusion** | ||
|
0.9% Sodium Chloride Injection, USP |
up to 24 hours |
up to 6 days |
|
0.45% Sodium Chloride Injection, USP |
up to 24 hours |
up to 6 days |
|
5% Dextrose Injection, USP |
up to 24 hours |
up to 36 hours |
|
5% Dextrose and 0.9% Sodium Chloride Injection, USP |
up to 24 hours |
up to 36 hours |
Storage of Undiluted Cyclophosphamide Solution (Multiple-Dose Vial):
After first use, store partially used multiple-dose vial in the original carton refrigerated at 2°C to 8°C (36ºF to 46°F) for up to 28 days or at room temperature at 20°C to 25°C (68ºF to 77°F) for up to 7 days. Discard unused portion.
During or immediately after FRINDOVYX administration, administer adequate amounts of fluid to reduce the risk of urinary tract toxicity (2.1).
Malignant Diseases: Adult and Pediatric Patients (2.2)
- Intravenous: Initial course for patients with no hematologic deficiency: 40 mg/kg to 50 mg/ kg in divided doses over 2 to 5 days. Other regimens include 10 mg/kg to 15 mg/kg given every 7 to 10 days or 3 mg/kg to 5 mg/kg twice weekly. (2.2)
- See full prescribing information for instructions on preparation, handling, and administration. (2.3)
DESCRIPTION SECTION
11 DESCRIPTION
Cyclophosphamide is an alkylating drug. It is an antineoplastic drug chemically related to the nitrogen mustards. The chemical name for cyclophosphamide is (±)-2-[Bis(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide monohydrate, and has the following structural formula:
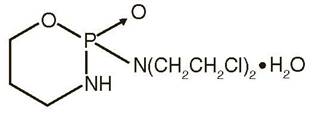
Cyclophosphamide is a white crystalline powder with the molecular formula C7H15Cl2N2O2 P•H2O and a molecular weight of 279.1. Cyclophosphamide is soluble in water, saline, or ethanol. The pKa of cyclophosphamide is 5.7.
FRINDOVYX is a clear, colorless to slight yellow sterile solution available as 500 mg/mL, 1 g/2 mL, and 2 g/4 mL in multiple dose vial for dilution prior to intravenous administration.
- 500 mg/mL vial contains 534.5 mg cyclophosphamide monohydrate, equivalent to 500 mg cyclophosphamide and 500 mg dehydrated alcohol (equivalent to 63% v/v).
- 1 g/2 mL vial contains 1069.0 mg cyclophosphamide monohydrate equivalent to 1 g cyclophosphamide and 1000 mg dehydrated alcohol (equivalent to 63% v/v).
- 2 g/4 mL vial contains 2138.0 mg cyclophosphamide monohydrate, equivalent to 2 g cyclophosphamide and 2000 mg dehydrated alcohol (equivalent to 63% v/v).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient of the following:
Myelosuppression, Immunosuppression, and Infections
● Inform patients of the possibility of myelosuppression, immunosuppression, and infections (sometimes fatal). Explain the need for routine blood cell counts. Instruct patients to monitor their temperature frequently and immediately report any occurrence of fever [see Warnings and Precautions (5.1)].
Urinary Tract and Renal Toxicity
● Advise the patient to report urinary symptoms (patients should report if their urine has turned a pink or red color) and the need for increasing fluid intake and frequent voiding [see Warnings and Precautions (5.2)].
Cardiotoxicity
● Inform patients of the possibility of cardiotoxicity (which may be fatal).
● Advise patients to contact a health care professional immediately for any of the following: new onset or worsening shortness of breath, cough, swelling of the ankles/legs, palpitations, weight gain of more than 5 pounds in 24 hours, dizziness or loss of consciousness [see Warnings and Precautions (5.3)].
Pulmonary Toxicity
● Warn patients of the possibility of developing non-infectious pneumonitis. Advise patients to report promptly any new or worsening respiratory symptoms [see Warnings and Precautions (5.4)].
Secondary Malignancies
● Inform patients that there is an increased risk of secondary malignancies with FRINDOVYX [see Warnings and Precautions (5.5)].
Alcohol Content
● Explain to patients the possible effects of the alcohol content in FRINDOVYX, including possible effects on the central nervous system. Patients in whom alcohol should be avoided or minimized should consider the alcohol content of FRINDOVYX. Alcohol could impair their ability to drive or use machines immediately after infusion [see Warnings and Precautions (5.7)].
Embryo-Fetal Toxicity
● Inform female patients of the risk to a fetus and potential loss of pregnancy. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1)].
● Advise female patients of reproductive potential to use effective contraception during treatment and for up to 1 year after completion of therapy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1, 8.3)].
● Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 4 months after completion of therapy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1, 8.3)].
Lactation
● Advise lactating women not to breastfeed during treatment and for 1 week after the last dose of FRINDOVYX [see Use in Specific Populations (8.2)].
Infertility
● Advise males and females of reproductive potential that FRINDOVYX may impair fertility [see Warnings and Precautions (5.9) and Use in Specific Populations (8.3, 8.4)].
Common Adverse Reactions
● Explain to patients that side effects such as nausea, vomiting, stomatitis, impaired wound healing, amenorrhea, premature menopause, sterility and hair loss may be associated with cyclophosphamide administration. Other undesirable effects (including, e.g., dizziness, blurred vision, visual impairment) could affect the ability to drive or use machines [see Adverse Reactions (6.1)].
Hydration and Important Administration Instructions
● Advise the patients that during or immediately after the administration, adequate amounts of fluid are required to reduce the risk of urinary tract toxicity [see Dosage and Administration (2.1)].
|
** Manufactured for:** |
|

