Sun Bum 50 Mineral Sunscreen 1 OZ
Sun Bum 50 Mineral Sunscreen 1 OZ
3c483d04-8942-5269-e063-6294a90a3881
HUMAN OTC DRUG LABEL
Aug 13, 2025
Sun Bum, LLC
DUNS: 028642574
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Zinc Oxide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (26)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Primary Artwork
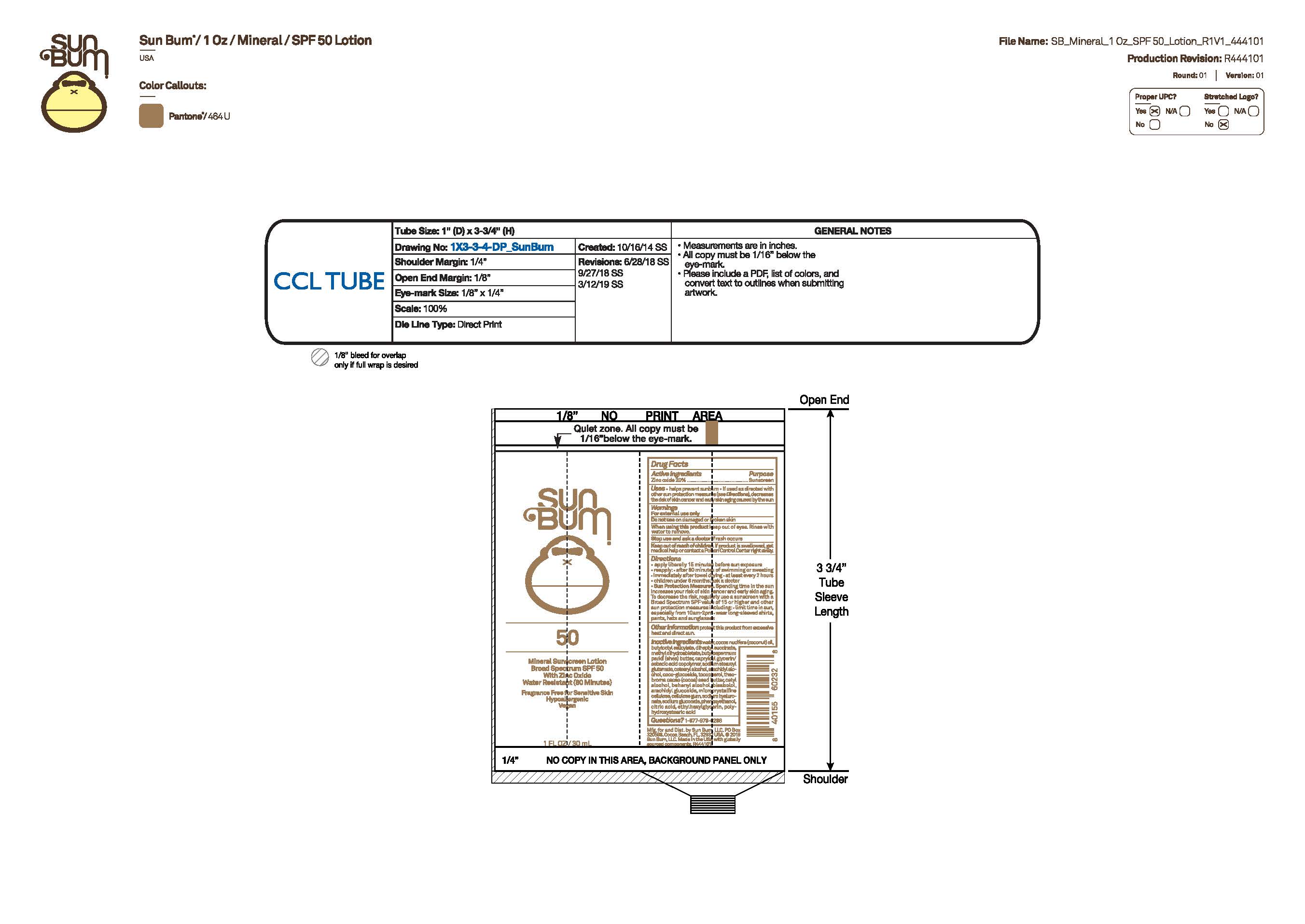
INDICATIONS & USAGE SECTION
Uses
• helps prevent sunburn • if used as directed with other sun protection measures, (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients
Zinc Oxide 20%
OTC - PURPOSE SECTION
Purpose
Sunscreen
WARNINGS SECTION
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Warnings
Keep Out Of Reach Of Children
INSTRUCTIONS FOR USE SECTION
Directions
• apply liberally 15 minutes before sun exposure
• reapply: • after 80 minutes of swimming or sweating • immediately after towel drying • at least every 2 hours
• children under 6 months of age: ask a doctor
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m.- 2 p.m.
• wear long-sleeved shirts, pants, hats and sunglasses
DOSAGE & ADMINISTRATION SECTION
Directions
• apply liberally 15 minutes before sun exposure
OTHER SAFETY INFORMATION
Other Information
protect this product from excessive heat and direct sun
INACTIVE INGREDIENT SECTION
Inactive Ingredients
METHYL DIHYDROABIETATE, TOCOPHEROL, POLYHYDROXYSTEARIC ACID, CITRIC ACID, BUTYROSPERMUM PARKII (SHEA) BUTTER, COCO-GLUCOSIDE, BUTYLOCTYL SALICYLATE, COCOS NUCIFERA (COCONUT) OIL, THEOBROMA CACAO (COCOA) SEED BUTTER, ETHYLHEXYLGLYCERIN, WATER, DIHEPTYL SUCCINATE, CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER, SODIUM STEAROYL GLUTAMATE, CETEARYL ALCOHOL, ARACHIDYL ALCOHOL, CETYL ALCOHOL, BEHENYL ALCOHOL, BISABOLOL, ARACHIDYL GLUCOSIDE, MICROCRYSTALLINE CELLULOSE, CELLULOSE GUM, SODIUM HYALURONATE, SODIUM GLUCONATE, PHENOXYETHANOL
OTC - QUESTIONS SECTION
Questions?
1-877-978-6286
