Triamcinolone Acetonide
Triamcinolone Acetonide Ointment USP, 0.025%, 0.1%, 0.5%
93041e9c-3320-4617-b1c1-68c95bf6b770
HUMAN PRESCRIPTION DRUG LABEL
Feb 4, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Triamcinolone Acetonide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and anti-pruritic agents. Triamcinolone acetonide is designated chemically as pregna-1,4-diene-3,20-dione, 9-fluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (11β,16α)-. C24H31FO6, M.W. 434.51; CAS Reg. No. 76-25-5.
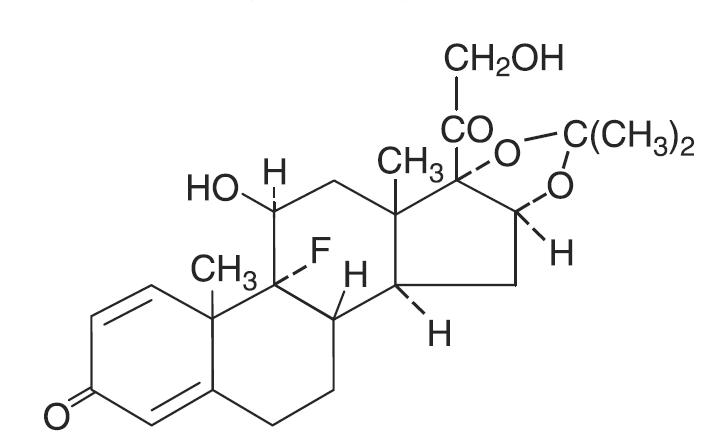
Each gram of Triamcinolone Acetonide Ointment USP, 0.025%, 0.1% or 0.5% contains 0.25 mg, 1 mg or 5 mg triamcinolone acetonide, respectively, in an ointment base of light mineral oil and white petrolatum.
