Regadenoson
These highlights do not include all the information needed to use REGADENOSON safely and effectively. See full prescribing information for REGADENOSON . REGADENOSON injection, for intravenous use Initial U.S. Approval: 2008
af131207-097f-3901-e711-fba004944076
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Apotex Corp.
DUNS: 845263701
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
regadenoson
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Regadenoson Injection 0.4 mg/5 mL (0.08 mg/mL) - Carton label
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
APOTEX CORP., NDC No. 60505-6116-0
Regadenoson Injection
0.4 mg/5 mL (0.08 mg/mL)
Rx Only

DESCRIPTION SECTION
11 DESCRIPTION
Regadenoson is an A2A adenosine receptor agonist that is a coronary vasodilator [see Clinical Pharmacology (12.1)]. Regadenoson is chemically described as adenosine, 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]. Its structural formula is:
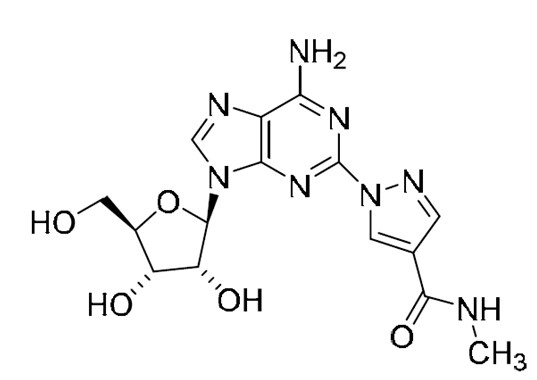
The molecular formula for regadenoson is C15H18N8O5 and its molecular weight is 390.35 g/mol.
Regadenoson is a sterile, nonpyrogenic solution for intravenous injection. The solution is clear and colorless. Each 1 mL in the 5 mL pre-filled syringe contains 0.08 mg regadenoson on an anhydrous basis, 10.9 mg dibasic sodium phosphate dihydrate, 5.4 mg monobasic sodium phosphate monohydrate, 150 mg propylene glycol, 1 mg edetate disodium dihydrate, and Water for Injection, with pH between 6.3 and 7.7.
