Triamcinolone Acetonide
Triamcinolone Acetonide Dental Paste USP, 0.1%
b353c8b1-9088-4969-8199-37876f2461cc
HUMAN PRESCRIPTION DRUG LABEL
Sep 15, 2025
Sun Pharmaceutical Industries, Inc.
DUNS: 146974886
Taro Pharmaceuticals U.S.A., Inc.
DUNS: 145186370
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Triamcinolone Acetonide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
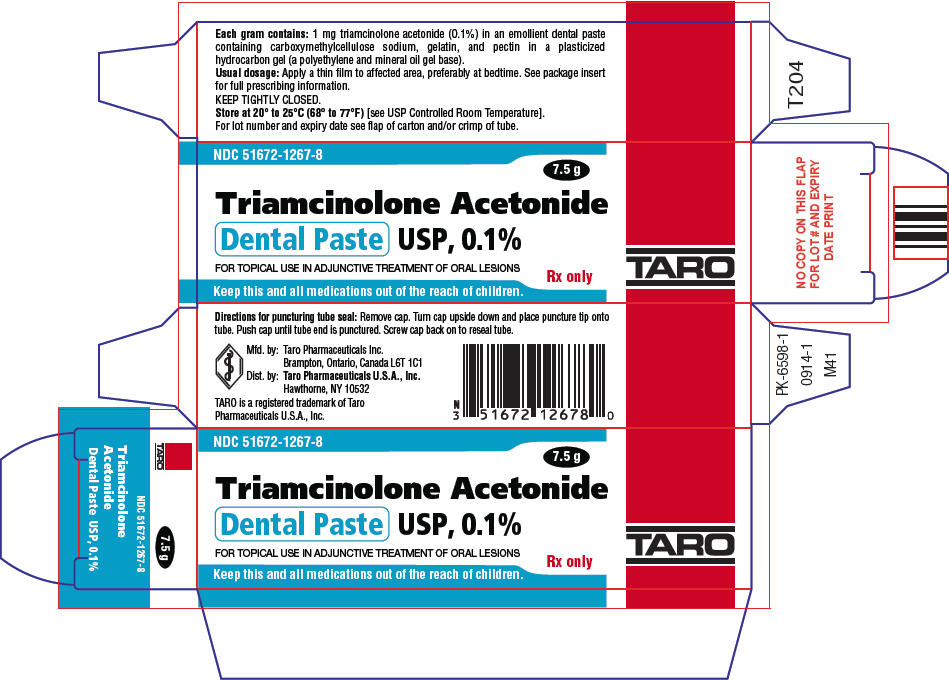
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 7.5 g Tube Carton
NDC 51672-1267-8
7.5 g
Triamcinolone Acetonide
** Dental Paste USP, 0.1%**
FOR TOPICAL USE IN ADJUNCTIVE TREATMENT OF ORAL LESIONS
Rx only
Keep this and all medications out of the reach of children.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
The following local adverse reactions may occur with corticosteroid-containing dental pastes: burning, itching, irritation, dryness, blistering or peeling not present prior to therapy, perioral dermatitis, allergic contact dermatitis, maceration of the oral mucosa, secondary infection, and atrophy of the oral mucosa.
Also, seePRECAUTIONSfor potential effects of systemic absorption.
To report SUSPECTED ADVERSE REACTINS, contact Sun Pharmaceutical Industries, Inc., at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
DESCRIPTION
Triamcinolone Acetonide Dental Paste USP, 0.1%, contains the corticosteroid triamcinolone acetonide in an adhesive vehicle suitable for application to oral tissues. Triamcinolone acetonide is designated chemically as 9-fluoro-11β, 16α, 17, 21-tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic 16, 17-acetal with acetone. The structural formula of triamcinolone acetonide is as follows:

C 24H 31FO 6 MW 434.50
Each gram of triamcinolone acetonide dental paste contains 1 mg triamcinolone acetonide in an emollient dental paste containing carboxymethylcellulose sodium, gelatin, and pectin in a plasticized hydrocarbon gel (a polyethylene and mineral oil gel base).
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Like other topical corticosteroids, triamcinolone acetonide has anti- inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A 2inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2.
Pharmacokinetics
The extent of absorption through the oral mucosa is determined by multiple factors including the vehicle, the integrity of the mucosal barrier, the duration of therapy, and the presence of inflammation and/or other disease processes. Once absorbed through the mucous membranes, the disposition of corticosteroids is similar to that of systemically administered corticosteroids. Corticosteroids are bound to the plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys; some corticosteroids and their metabolites are also excreted into the bile.
HOW SUPPLIED SECTION
HOW SUPPLIED
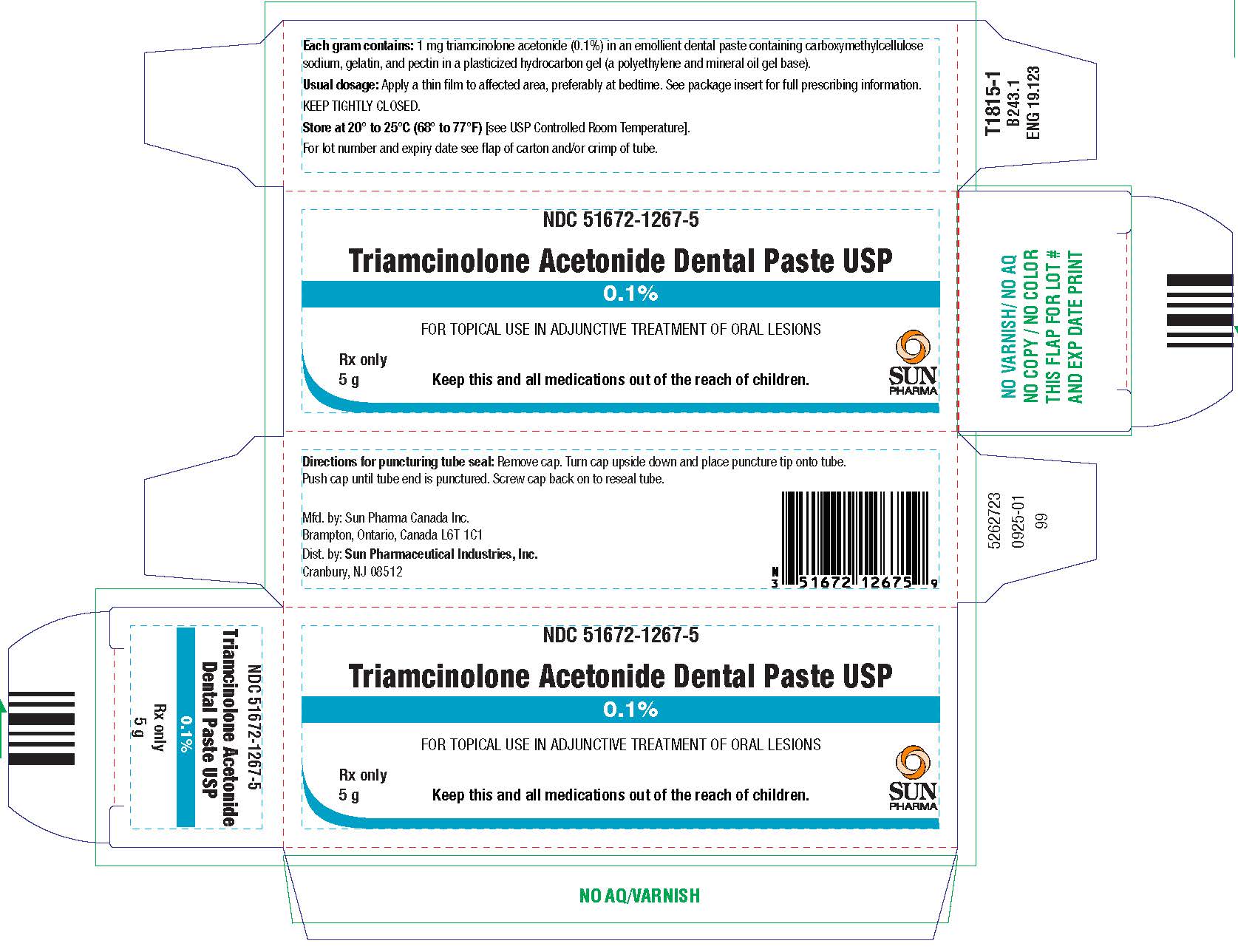
Triamcinolone Acetonide Dental Paste USP, 0.1% is supplied in tubes containing 5 g of dental paste (NDC 51672–1267-5) and 7.5 g of dental paste (NDC 51672-1267-8).
Storage
Keep tightly closed.Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room Temperature].
SPL UNCLASSIFIED SECTION
Mfd. by: Sun Pharma Canada Inc.
Brampton, Ontario, Canada L6T 1C1
Dist. by:Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Revised: September 2025
5264365-0925-01 364
