SOVEREIGN SILVER MULTI-SYMPTOM SORE THROAT
b5ef5d51-61d6-4a46-b68d-95150bb06bbb
HUMAN OTC DRUG LABEL
May 22, 2025
Natural Immunogenics Corp.dba SOVEREIGN NATURALS
DUNS: 048744085
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ARGENTUM NITRICUM
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
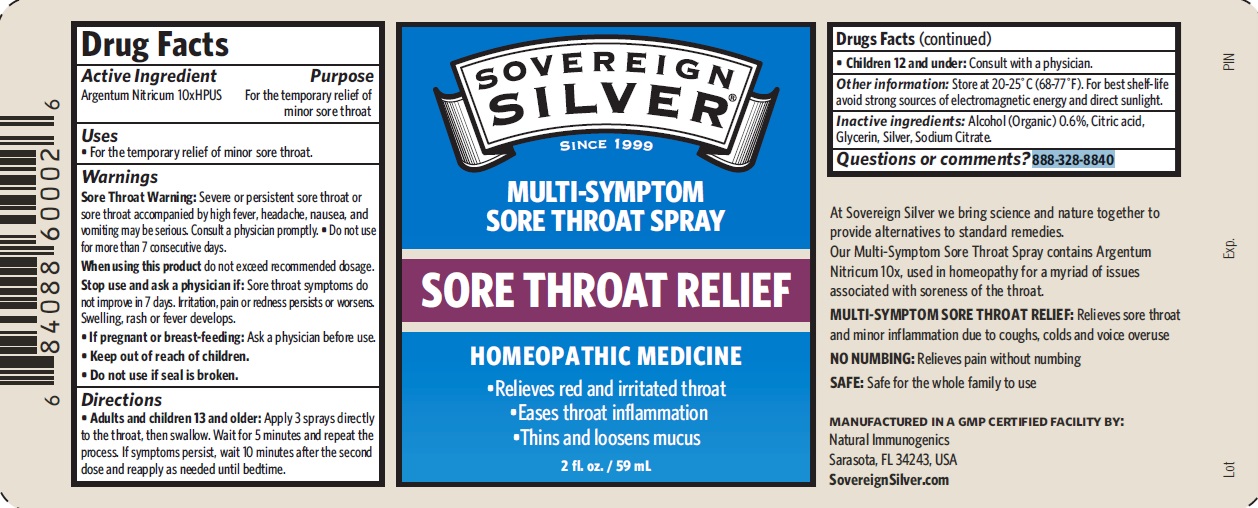
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product label
INDICATIONS & USAGE SECTION
Uses
For the temporary relief of minor sore throat.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient Purpose
Argentum Nitricum 10xHPUS For the temporary relief of minor sore throat
OTC - PURPOSE SECTION
WARNINGS SECTION
Warnings
*Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a physician promptly. • Do not use for more than 7 consecutive days.
*When using this productdo not exceed recommended dosage.
*Stop use and ask a physician if: Sore throat symptoms do not improve in 7 days. Irritation, pain or redness persists or worsens.Swelling, rash or fever develops.
*If pregnant or breast-feeding: Ask a physician before use.
*Keep out of reach of children.
*Do not use if seal is broken.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
DOSAGE & ADMINISTRATION SECTION
Directions
*Adults and children 13 and older: Apply 3 sprays directly to the throat, then swallow. Wait for 5 minutes and repeat the process. If symptoms persist, wait 10 minutes after the second dose and reapply as needed until bedtime. *Children 12 and under: Consult with a physician.
SPL UNCLASSIFIED SECTION
Questions or comments?
888-328-8840
INACTIVE INGREDIENT SECTION
Inactive ingredients
Alcohol (Organic) 0.6%, Citric acid, Glycerin, Silver, Sodium Citrate.
