N12
N12
0c54fc72-792f-0b9b-e063-6394a90ac2f0
HUMAN OTC DRUG LABEL
May 15, 2025
Apex Energetics Inc.
DUNS: 195816384
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ARSENIC TRIOXIDE, BAPTISIA TINCTORIA ROOT, SUS SCROFA BONE MARROW, BRYONIA ALBA ROOT, ECHINACEA ANGUSTIFOLIA, ECHINACEA PURPUREA, OLEA EUROPAEA FLOWER, ULEX EUROPAEUS, FLOWER, LACHESIS MUTA VENOM, LEVISTICUM OFFICINALE, TREPONEMIC SKIN CANKER HUMAN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (27)
Drug Labeling Information
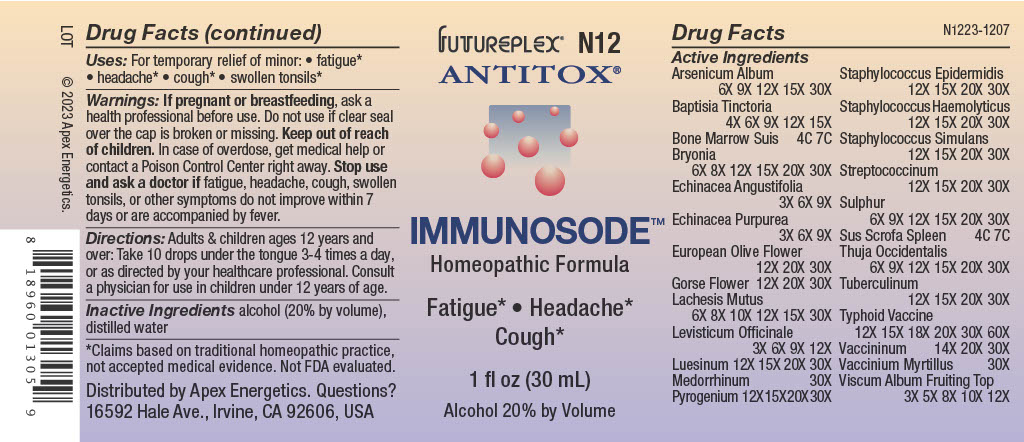
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FUTUREPLEX® N12
ANTITOX®
IMMUNOSODE™
Homeopathic Formula
Fatigue* Headaches*
Cough*
1 fl oz (30 mL)
Alcohol 20% by Volume
INDICATIONS & USAGE SECTION
Uses:
For temporary relief of minor:
fatigue*
headache*
cough*
swollen tonsils*
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
OTC - ACTIVE INGREDIENT SECTION
|
Active Ingredients | |
|
Arsenicum Album |
6X 9X 12X 15X 30X |
|
Baptisia Tinctoria |
4X 6X 9X 12X 15X |
|
Bone Marrow Suis |
4C 7C |
|
Bryonia |
6X 8X 12X 15X 20X 30X |
|
Echinacea Angustifolia |
3X 6X 9X |
|
Echinacea Purpurea |
3X 6X 9X |
|
European Olive Flower |
12X 20X 30X |
|
Gorse Flower |
12X 20X 30X |
|
Lachesis Mutus |
6X 8X 10X 12X 15X 30X |
|
Levisticum Officinale |
3X 6X 9X 12X |
|
Luesinum |
12X 15X 20X 30X |
|
Medorrhinum |
30X |
|
Pyrogenium |
12X 15X 20X 30X |
|
Staphylococcus Epidermidis |
12X 15X 20X 30X |
|
Staphylococcus Haemolyticus |
12X 15X 20X 30X |
|
Staphylococcus Simulans |
12X 15X 20X 30X |
|
Streptococcinum |
12X 15X 20X 30X |
|
Sulphur |
6X 9X 12X 15X 20X 30X |
|
Sus Scrofa Spleen |
4C 7C |
|
Thuja Occidentalis |
6X 9X 12X 15X 20X 30X |
|
Tuberculinum |
12X 15X 20X 30X |
|
Typhoid Vaccine |
12X 15X 18X 20X 30X 60X |
|
Vaccininum |
14X 20X 30X |
|
Vaccinium Myrtillus |
30X |
|
Viscum Album Fruiting Top |
3X 5X 8X 10X 12X |
WARNINGS SECTION
Warnings:
If pregnant or breastfeeding, ask a health professional before use.
Do not use if clear seal over the cap is broken or missing.
**Keep out of reach of children.**In case of overdose, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if fatigue, headaches, cough, swollen tonsils, or other symptoms do not improve within 7 days or are accompanied by fever.
DOSAGE & ADMINISTRATION SECTION
Directions:
Adults & children ages 12 years and over: Take 10 drops under the tongue 3-4 times a day, or as directed by your healthcare professional. Consult a physician for use in children under 12 years of age.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
alcohol (20% by volume), distilled water
OTC - QUESTIONS SECTION
Distributed by Apex Energetics. Questions?
16592 Hale Ave., Irvine, CA 92606, USA
