Phenytoin
These highlights do not include all the information needed to use PHENYTOIN ORAL SUSPENSION safely and effectively. See full prescribing information for PHENYTOIN ORAL SUSPENSION. PHENYTOIN oral suspension Initial U.S. Approval: 1953
f9fe4c92-2761-4cb4-9cf3-7066e240e014
HUMAN PRESCRIPTION DRUG LABEL
Sep 17, 2025
American Health Packaging
DUNS: 929561009
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Phenytoin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel – Cup – 100 mg per 4 mL – 4 mL

Rx Only
NDC 60687-275-62
Phenytoin
Oral Suspension, USP
100 mg/4 mL
Delivers 4 mL
SHAKE WELL
NOT FOR PARENTERAL USE
Protect from freezing. See package insert for full
prescribing information and storage.
For Institutional Use Only.
American Health Packaging
Columbus, OH 43217
0427566/0221
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Phenytoin oral suspension is indicated for the treatment of tonic-clonic (grand mal) and psychomotor (temporal lobe) seizures.
Phenytoin oral suspension is indicated for the treatment of tonic-clonic (grand mal) and psychomotor (temporal lobe) seizures. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Phenytoin oral suspension is contraindicated in patients with:
- A history of hypersensitivity to phenytoin, its inactive ingredients, or other hydantoins [see Warnings and Precautions (5.5)]. Reactions have included angioedema.
- A history of prior acute hepatotoxicity attributable to phenytoin [see Warnings and Precautions (5.8)].
- Coadministration with delavirdine because of the potential for loss of virologic response and possible resistance to delavirdine or to the class of non-nucleoside reverse transcriptase inhibitors.
- Hypersensitivity to phenytoin, its ingredients, or other hydantoins (4, 5.5)
- A history of prior acute hepatotoxicity attributable to phenytoin (4, 5.8)
- Coadministration with delavirdine (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Withdrawal Precipitated Seizure, Status Epilepticus [see Warnings and Precautions (5.1)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.2)]
- Serious Dermatologic Reactions [see Warnings and Precautions (5.3)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity [see Warnings and Precautions (5.4)]
- Hypersensitivity [see Warnings and Precautions (5.5)]
- Cardiac Effects [see Warnings and Precautions (5.6)]
- Angioedema [see Warnings and Precautions (5.7)]
- Hepatic Injury [see Warnings and Precautions (5.8)]
- Hematopoietic Complications [see Warnings and Precautions (5.9)]
- Effects on Vitamin D and Bone [see Warnings and Precautions (5.10)]
- Exacerbation of Porphyria [see Warnings and Precautions (5.12)]
- Teratogenicity and Other Harm to the Newborn [see Warnings and Precautions (5.13)]
- Hyperglycemia [see Warnings and Precautions (5.14)]
The following adverse reactions associated with the use of phenytoin were identified in clinical studies or postmarketing reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Allergic reactions in the form of rash and rarely more serious forms and DRESS have been observed as has angioedema [see Warnings and Precautions (5.3, 5.4, 5.7)]. Anaphylaxis has also been reported.
There have also been reports of coarsening of facial features, systemic lupus erythematosus, periarteritis nodosa, and immunoglobulin abnormalities.
Digestive System: Acute hepatic failure, toxic hepatitis, liver damage, nausea, vomiting, constipation, enlargement of the lips, and gingival hyperplasia.
Hematologic and Lymphatic System: Hematopoietic complications, some fatal, have occasionally been reported in association with administration of phenytoin. These have included thrombocytopenia, leukopenia, granulocytopenia, agranulocytosis, and pancytopenia with or without bone marrow suppression. While macrocytosis and megaloblastic anemia have occurred, these conditions usually respond to folic acid therapy. Lymphadenopathy including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin's disease have been reported [see Warnings and Precautions (5.9)].
Laboratory Test Abnormality: Phenytoin may decrease serum concentrations of thyroid hormone (T4 and T3), sometimes with an accompanying increase in thyroid-stimulating hormone (TSH), but usually in the absence of clinical hypothyroidism. Phenytoin may also produce lower than normal values for dexamethasone or metyrapone tests. Phenytoin may cause increased serum levels of glucose [see Warnings and Precautions (5.14)], alkaline phosphatase, and gamma glutamyl transpeptidase (GGT).
Nervous System: The most common adverse reactions encountered with phenytoin therapy are nervous system reactions and are usually dose-related. Reactions include nystagmus, ataxia, slurred speech, decreased coordination, somnolence, and mental confusion. Dizziness, vertigo, insomnia, transient nervousness, motor twitchings, paresthesias, and headaches have also been observed. There have also been rare reports of phenytoin-induced dyskinesias, including chorea, dystonia, tremor and asterixis, similar to those induced by phenothiazine and other neuroleptic drugs. Cerebellar atrophy has been reported, and appears more likely in settings of elevated phenytoin levels and/or long-term phenytoin use [see Warnings and Precautions (5.15)].
A predominantly sensory peripheral polyneuropathy has been observed in patients receiving long-term phenytoin therapy.
Skin and Appendages: Dermatological manifestations sometimes accompanied by fever have included scarlatiniform or morbilliform rashes. A morbilliform rash (measles-like) is the most common; other types of dermatitis are seen more rarely. Other more serious forms which may be fatal have included bullous, exfoliative or purpuric dermatitis, acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, and toxic epidermal necrolysis [see Warnings and Precautions (5.3)]. There have also been reports of hypertrichosis and urticaria.
Special Senses: Altered taste sensation including metallic taste.
Urogenital: Peyronie's disease
The most common adverse reactions are nervous system reactions, including nystagmus, ataxia, slurred speech, decreased coordination, somnolence, and mental confusion. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Taro Pharmaceuticals U.S.A., Inc. at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Phenytoin is extensively bound to plasma proteins and is prone to competitive displacement. Phenytoin is primarily metabolized by the hepatic cytochrome P450 enzyme CYP2C9 and to a lesser extent by CYP2C19 and is particularly susceptible to inhibitory drug interactions because it is subject to saturable metabolism. Inhibition of metabolism may produce significant increases in circulating phenytoin concentrations and enhance the risk of drug toxicity. Monitoring of phenytoin serum levels is recommended when a drug interaction is suspected.
Phenytoin is a potent inducer of hepatic drug-metabolizing enzymes.
7.1 Drugs that Affect Phenytoin Concentrations
Table 2 includes commonly occurring drug interactions that affect phenytoin concentrations. However, this list is not intended to be inclusive or comprehensive. Individual prescribing information from relevant drugs should be consulted.
The addition or withdrawal of these agents in patients on phenytoin therapy may require an adjustment of the phenytoin dose to achieve optimal clinical outcome.
Table 2: Drugs That Affect Phenytoin Concentrations|
Interacting Agent |
Examples |
|---|---|
| |
|
Drugs that may increase phenytoin serum levels | |
|
Antiepileptic drugs |
Ethosuximide, felbamate, oxcarbazepine, methsuximide, topiramate |
|
Azoles |
Fluconazole, ketoconazole, itraconazole, miconazole, voriconazole |
|
Antineoplastic agents |
Capecitabine, fluorouracil |
|
Antidepressants |
Fluoxetine, fluvoxamine, sertraline |
|
Gastric acid reducing agents |
H 2 antagonists (cimetidine), omeprazole |
|
Sulfonamides |
Sulfamethizole, sulfaphenazole, sulfadiazine, sulfamethoxazole-trimethoprim |
|
Other |
Acute alcohol intake, amiodarone, chloramphenicol, chlordiazepoxide, disulfiram, estrogen, fluvastatin, isoniazid, methylphenidate, phenothiazines, salicylates, ticlopidine, tolbutamide, trazodone, warfarin |
|
Drugs that may decrease phenytoin serum levels | |
|
Antacids * |
Calcium carbonate, aluminum hydroxide, magnesium hydroxide Prevention or Management: Phenytoin and antacids should not be taken at the same time of day |
|
Antineoplastic agents (usually in combination) |
Bleomycin, carboplatin, cisplatin, doxorubicin, methotrexate |
|
Antiviral agents |
Fosamprenavir, nelfinavir, ritonavir |
|
Antiepileptic drugs |
Carbamazepine, vigabatrin |
|
Other |
Chronic alcohol abuse, diazepam, diazoxide, folic acid, reserpine, rifampin, St. John's wort †, sucralfate, theophylline |
|
Drugs that may either increase or decrease phenytoin serum levels | |
|
Antiepileptic drugs |
Phenobarbital, valproate sodium ‡, valproic acid ‡ |
7.2 Drugs Affected by Phenytoin
Table 3 includes commonly occurring drug interactions affected by phenytoin. However, this list is not intended to be inclusive or comprehensive. Individual drug package inserts should be consulted.
The addition or withdrawal of phenytoin during concomitant therapy with these agents may require adjustment of the dose of these agents to achieve optimal clinical outcome.
Table 3: Drugs Affected by Phenytoin|
Interacting Agent |
Examples |
|---|---|
| |
|
Drugs whose efficacy is impaired by phenytoin | |
|
Azoles |
Fluconazole, ketoconazole, itraconazole, posaconazole, voriconazole |
|
Antineoplastic agents |
Irinotecan, paclitaxel, teniposide |
|
Delavirdine |
Phenytoin can substantially reduce the concentrations of delavirdine. This can lead to loss of virologic response and possible resistance [see Contraindications (4)]. |
|
Neuromuscular blocking agents |
Cisatracurium, pancuronium, rocuronium and vecuronium: resistance to the neuromuscular blocking action of the nondepolarizing neuromuscular blocking agents has occurred in patients chronically administered phenytoin. Whether or not phenytoin has the same effect on other non-depolarizing agents is unknown. Prevention or Management: Patients should be monitored closely for more rapid recovery from neuromuscular blockade than expected, and infusion rate requirements may be higher. |
|
Warfarin |
Increased and decreased PT/INR responses have been reported when phenytoin is coadministered with warfarin |
|
Other |
Corticosteroids, doxycycline, estrogens, furosemide, oral contraceptives, paroxetine, quinidine, rifampin, sertraline, theophylline, and vitamin D |
|
Drugs whose level is decreased by phenytoin | |
|
Anticoagulants |
Apixaban, dabigatran, edoxaban, rivaroxaban |
|
Antiepileptic drugs * |
Carbamazepine, felbamate, lacosamide, lamotrigine, topiramate, oxcarbazepine |
|
Antilipidemic agents |
Atorvastatin, fluvastatin, simvastatin |
|
Antiplatelets |
Ticagrelor |
|
Antiviral agents |
Efavirenz, lopinavir/ritonavir, indinavir, nelfinavir, ritonavir, saquinavir Fosamprenavir: phenytoin when given with fosamprenavir alone may decrease the concentration of amprenavir, the active metabolite. Phenytoin when given with the combination of fosamprenavir and ritonavir may increase the concentration of amprenavir |
|
Calcium channel blockers |
Nifedipine, nimodipine, nisoldipine, verapamil |
|
Other |
Albendazole (decreases active metabolite), chlorpropamide, clozapine, cyclosporine, digoxin, disopyramide, folic acid, methadone, mexiletine, praziquantel, quetiapine |
7.3 Hyperammonemia with Concomitant Use of Valproate
Concomitant administration of phenytoin and valproate has been associated with an increased risk of valproate-associated hyperammonemia. Patients treated concomitantly with these two drugs should be monitored for signs and symptoms of hyperammonemia.
7.4 Drug Enteral Feeding/Nutritional Preparations Interaction
Literature reports suggest that patients who have received enteral feeding preparations and/or related nutritional supplements have lower than expected phenytoin serum levels. It is therefore suggested that phenytoin not be administered concomitantly with an enteral feeding preparation. More frequent serum phenytoin level monitoring may be necessary in these patients.
7.5 Drug/Laboratory Test Interactions
Care should be taken when using immunoanalytical methods to measure serum phenytoin concentrations.
Multiple drug interactions because of extensive plasma protein binding, saturable metabolism and potent induction of hepatic enzymes (7.1, 7.2).
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Phenytoin oral suspension is available as a 125 mg phenytoin/5 mL oral suspension of orange color with an orange-vanilla flavor.
Phenytoin oral suspension is available as a 125 mg phenytoin/5 mL oral suspension. (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in
women exposed to antiepileptic drugs (AEDs), such as phenytoin, during
pregnancy. Physicians are advised to recommend that pregnant patients taking
phenytoin enroll in the North American Antiepileptic Drug (NAAED) Pregnancy
Registry. This can be done by calling the tollfree number 1-888-233-2334, and
must be done by patients themselves. Information on the registry can also be
found at the website http://www.aedpregnancyregistry.org/.
Risk Summary
In humans, prenatal exposure to phenytoin may increase the risks for
congenital malformations and other adverse developmental outcomes. Prenatal
phenytoin exposure is associated with an increased incidence of major
malformations, including orofacial clefts and cardiac defects. In addition,
the fetal hydantoin syndrome, a pattern of abnormalities including dysmorphic
skull and facial features, nail and digit hypoplasia, growth abnormalities
(including microcephaly), and cognitive deficits has been reported among
children born to epileptic women who took phenytoin alone or in combination
with other antiepileptic drugs during pregnancy [see Data]. There have been
several reported cases of malignancies, including neuroblastoma, in children
whose mothers received phenytoin during pregnancy.
Administration of phenytoin to pregnant animals resulted in an increased incidence of fetal malformations and other manifestations of developmental toxicity (including embryofetal death, growth impairment, and behavioral abnormalities) in multiple species at clinically relevant doses [see Data].
In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Disease-associated maternal risk
An increase in seizure frequency may occur during pregnancy because of altered
phenytoin pharmacokinetics. Periodic measurement of serum phenytoin
concentrations may be valuable in the management of pregnant women as a guide
to appropriate adjustment of dosage [see Dosage and Administration (2.4, 2.8)]. However, postpartum restoration of the original dosage will probably be
indicated [see Clinical Pharmacology (12.3)].
Fetal/Neonatal Adverse Reactions
A potentially life-threatening bleeding disorder related to decreased levels
of vitamin K-dependent clotting factors may occur in newborns exposed to
phenytoin in utero. This drug-induced condition can be prevented with vitamin
K administration to the mother before delivery and to the neonate after birth.
Data
Human Data
Meta-analyses using data from published observational studies and registries
have estimated an approximately 2.4-fold increased risk for any major
malformation in children with prenatal phenytoin exposure compared to
controls. An increased risk of heart defects, facial clefts, and digital
hypoplasia has been reported. The fetal hydantoin syndrome is a pattern of
congenital anomalies including craniofacial anomalies, nail and digital
hypoplasia, prenatal-onset growth deficiency, and neurodevelopmental
deficiencies.
Animal Data
Administration of phenytoin to pregnant rats, rabbits, and mice during
organogenesis resulted in embryofetal death, fetal malformations, and
decreased fetal growth. Malformations (including craniofacial, cardiovascular,
neural, limb, and digit abnormalities) were observed in rats, rabbits, and
mice at doses as low as 100 mg/kg, 75 mg/kg, and 12.5 mg/kg, respectively.
8.2 Lactation
Risk Summary
Phenytoin is secreted in human milk. The developmental and health benefits of
breastfeeding should be considered along with the mother's clinical need for
phenytoin and any potential adverse effects on the breastfed infant from
phenytoin or from the underlying maternal condition.
8.4 Pediatric Use
Initially, 5 mg/kg/day in two or three equally divided doses, with subsequent dosage individualized to a maximum of 300 mg daily. A recommended daily maintenance dosage is usually 4 mg/kg to 8 mg/kg. Children over 6 years and adolescents may require the minimum adult dosage (300 mg/day) [see Dosage and Administration (2.3)].
8.5 Geriatric Use
Phenytoin clearance tends to decrease with increasing age [see Clinical Pharmacology (12.3)]. Lower or less frequent dosing may be required [see Dosage and Administration (2.7)].
8.6 Renal and/or Hepatic Impairment or Hypoalbuminemia
The liver is the chief site of biotransformation of phenytoin; patients with impaired liver function, elderly patients, or those who are gravely ill may show early signs of toxicity.
Because the fraction of unbound phenytoin is increased in patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of phenytoin serum levels should be based on the unbound fraction in those patients.
8.7 Use in Patients with Decreased CYP2C9 Function
Patients who are intermediate or poor metabolizers of CYP2C9 substrates (e.g., *1/*3, *2/*2, *3/*3) may exhibit increased phenytoin serum concentrations compared to patients who are normal metabolizers (e.g., *1/*1). Thus, patients who are known to be intermediate or poor metabolizers may ultimately require lower doses of phenytoin to maintain similar steady-state concentrations compared to normal metabolizers. If early signs of dose-related central nervous system (CNS) toxicity develop, serum concentrations should be checked immediately [see Clinical Pharmacology (12.5)].
- Pregnancy: Prenatal exposure may increase the risks for congenital malformations and other adverse developmental outcomes. (5.13, 8.1)
- Renal and/or Hepatic Impairment or Hypoalbuminemia: Monitor unbound phenytoin concentrations in these patients (8.6).
OVERDOSAGE SECTION
10 OVERDOSAGE
The lethal dose in pediatric patients is not known. The lethal dose in adults is estimated to be 2 grams to 5 grams. The initial symptoms are nystagmus, ataxia, and dysarthria. Other signs are tremor, hyperreflexia, lethargy, slurred speech, blurred vision, nausea, and vomiting. The patient may become comatose and hypotensive. Bradycardia and cardiac arrest have been reported [see Warnings and Precautions (5.6)]. Death is caused by respiratory and circulatory depression. There are marked variations among individuals with respect to phenytoin serum levels where toxicity may occur. Nystagmus, on lateral gaze, usually appears at 20 mcg/mL, ataxia at 30 mcg/mL, dysarthria and lethargy appear when the serum concentration is over 40 mcg/mL, but as high a concentration as 50 mcg/mL has been reported without evidence of toxicity. As much as 25 times the therapeutic dose has been taken to result in a serum concentration over 100 mcg/mL with complete recovery. Irreversible cerebellar dysfunction and atrophy have been reported.
Treatment: Treatment is nonspecific since there is no known antidote.
The adequacy of the respiratory and circulatory systems should be carefully observed and appropriate supportive measures employed. Hemodialysis can be considered since phenytoin is not completely bound to plasma proteins. Total exchange transfusion has been used in the treatment of severe intoxication in pediatric patients.
In acute overdosage the possibility of other CNS depressants, including alcohol, should be borne in mind.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism by which phenytoin exerts its therapeutic effect has not been established but is thought to involve the voltage-dependent blockade of membrane sodium channels resulting in a reduction in sustained high-frequency neuronal discharges.
12.3 Pharmacokinetics
Absorption
For phenytoin oral suspension, peak levels occur 1½ to 3 hours after
administration. Steady-state therapeutic levels are achieved at least 7 to 10
days (5 to 7 half-lives) after initiation of therapy with recommended doses of
300 mg/day. When serum level determinations are necessary, they should be
obtained at least 5 to 7 half-lives after treatment initiation, dosage change,
or addition or subtraction of another drug to the regimen so that equilibrium
or steady-state will have been achieved.
Distribution
Phenytoin is extensively bound to serum plasma proteins.
Elimination
The plasma half-life in man after oral administration of phenytoin averages 22
hours, with a range of 7 to 42 hours.
Metabolism
Phenytoin is primarily metabolized by the hepatic cytochrome P450 enzyme
CYP2C9 and to a lesser extent by CYP2C19. Because phenytoin is hydroxylated in
the liver by an enzyme system which is saturable at high serum levels, small
incremental doses may increase the half-life and produce very substantial
increases in serum levels, when these are in the upper range. The steady-state
level may be disproportionately increased, with resultant intoxication, from
an increase in dosage of 10% or more.
In most patients maintained at a steady dosage, stable phenytoin serum levels are achieved. There may be wide interpatient variability in phenytoin serum levels with equivalent dosages. Patients with unusually low levels may be noncompliant or hypermetabolizers of phenytoin. Unusually high levels result from liver disease, variant CYP2C9 and CYP2C19 alleles, or drug interactions which result in metabolic interference. The patient with large variations in phenytoin serum levels, despite standard doses, presents a difficult clinical problem. Serum level determinations in such patients may be particularly helpful. As phenytoin is highly protein bound, free phenytoin levels may be altered in patients whose protein binding characteristics differ from normal.
Excretion
Most of the drug is excreted in the bile as inactive metabolites which are
then reabsorbed from the intestinal tract and excreted in the urine. Urinary
excretion of phenytoin and its metabolites occurs partly with glomerular
filtration but, more importantly, by tubular secretion.
Specific Populations
Age: Geriatric Population:
Phenytoin clearance tends to decrease with increasing age (20% less in
patients over 70 years of age relative to that in patients 20 to 30 years of
age). Since phenytoin clearance is decreased slightly in elderly patients,
lower or less frequent dosing may be required [see Dosage and Administration (2.7)].
Sex/Race:
Gender and race have no significant impact on phenytoin pharmacokinetics.
Renal or Hepatic Impairment:
Increased fraction of unbound phenytoin in patients with renal or hepatic
disease, or in those with hypoalbuminemia has been reported.
Pregnancy:
It has been reported in the literature that the plasma clearance of phenytoin
generally increased during pregnancy, reached a peak in the third trimester
and returned to the level of pre-pregnancy after few weeks or months of
delivery.
Drug Interaction Studies
Phenytoin is primarily metabolized by the hepatic cytochrome P450 enzyme
CYP2C9 and to a lesser extent by CYP2C19.
Phenytoin is a potent inducer of hepatic drug-metabolizing enzymes [see Drug Interactions (7.1, 7.2)].
12.5 Pharmacogenomics
CYP2C9 activity is decreased in individuals with genetic variants such as the CYP2C92 and CYP2C93 alleles. Carriers of variant alleles, resulting in intermediate (e.g., *1/*3, *2/*2) or poor metabolism (e.g., *2/*3, *3/*3) have decreased clearance of phenytoin. Other decreased or nonfunctional CYP2C9 alleles may also result in decreased clearance of phenytoin (e.g., *5, *6, *8, *11).
The prevalence of the CYP2C9 poor metabolizer phenotype is approximately 2 to 3% in the White population, 0.5 to 4% in the Asian population, and <1% in the African American population. The CYP2C9 intermediate phenotype prevalence is approximately 35% in the White population, 24% in the African American population, and 15 to 36% in the Asian population [see Warnings and Precautions (5.3) and Use in Specific Populations (8.7)].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis[see Warnings and Precautions (5.9)]
In carcinogenicity studies, phenytoin was administered in the diet to mice (10
mg/kg/day, 25 mg/kg/day, or 45 mg/kg/day) and rats (25 mg/kg/day, 50
mg/kg/day, or 100 mg/kg/day) for 2 years. The incidences of hepatocellular
tumors were increased in male and female mice at the highest dose. No
increases in tumor incidence were observed in rats. The highest doses tested
in these studies were associated with peak serum phenytoin levels below human
therapeutic concentrations.
In carcinogenicity studies reported in the literature, phenytoin was administered in the diet for 2 years at doses up to 600 ppm (approximately 160 mg/kg/day) to mice and up to 2400 ppm (approximately 120 mg/kg/day) to rats. The incidences of hepatocellular tumors were increased in female mice at all but the lowest dose tested. No increases in tumor incidence were observed in rats.
Mutagenesis
Phenytoin was negative in the Ames test and in the in vitro clastogenicity
assay in Chinese hamster ovary (CHO) cells.
In studies reported in the literature, phenytoin was negative in the in vitro mouse lymphoma assay and the in vivo micronucleus assay in mouse. Phenytoin was clastogenic in the in vitro sister chromatid exchange assay in CHO cells.
Fertility
Phenytoin has not been adequately assessed for effects on male or female
fertility.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Phenytoin Oral Suspension USP, 125 mg phenytoin/5 mL is supplied as follows:
4 mL unit dose cups:
50 cups (5 x 10) NDC 60687-275-66
16.2 Storage and Handling
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from light. Do not freeze.
DO NOT USE IF SEAL IS BROKEN.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Medication Guide).
Administration Information
Advise patients taking phenytoin of the importance of adhering strictly to the
prescribed dosage regimen, and of informing the physician of any clinical
condition in which it is not possible to take the drug orally as prescribed,
e.g., surgery, etc.
Instruct patients to use an accurately calibrated measuring device when using this medication to ensure accurate dosing.
Withdrawal of Antiepileptic Drugs
Advise patients not to discontinue use of phenytoin without consulting with
their healthcare provider. Phenytoin should normally be gradually withdrawn to
reduce the potential for increased seizure frequency and status epilepticus
[see Warnings and Precautions (5.1)].
Suicidal Ideation and Behavior
Counsel patients, their caregivers, and families that AEDs, including
phenytoin, may increase the risk of suicidal thoughts and behavior and advise
them of the need to be alert for the emergence or worsening of symptoms of
depression, any unusual changes in mood or behavior, or the emergence of
suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern
should be reported immediately to healthcare providers [see Warnings and Precautions (5.2)].
Serious Dermatologic Reactions
Advise patients of the early signs and symptoms of severe cutaneous adverse
reactions and to report any occurrence immediately to a physician [see Warnings and Precautions (5.3)].
Potential Signs of Drug Reaction with Eosinophilia and Systemic Symptoms
(DRESS) and Other Systemic Reactions
Advise patients of the early toxic signs and symptoms of potential
hematologic, dermatologic, hypersensitivity, or hepatic reactions. These
symptoms may include, but are not limited to, fever, sore throat, rash, ulcers
in the mouth, easy bruising, lymphadenopathy, facial swelling, and petechial
or purpuric hemorrhage, and in the case of liver reactions, anorexia,
nausea/vomiting, or jaundice. Advise the patient that, because these signs and
symptoms may signal a serious reaction, that they must report any occurrence
immediately to a physician. In addition, advise the patient that these signs
and symptoms should be reported even if mild or when occurring after extended
use [see Warnings and Precautions (5.3, 5.4, 5.5, 5.8, 5.9)].
Cardiac Effects
Counsel patients that cases of bradycardia and cardiac arrest have been
reported, both at recommended phenytoin doses and levels, and in association
with phenytoin toxicity. Patients should report cardiac signs or symptoms to
their healthcare provider [see Warnings and Precautions (5.6) and Overdosage (10)].
Angioedema
Advise patients to discontinue phenytoin oral suspension and seek immediate
medical care if they develop signs or symptoms of angioedema, such as facial,
perioral, or upper airway swelling [see Warnings and Precautions (5.7)].
Effects of Alcohol Use and Other Drugs and Over-the-Counter Drug Interactions
Caution patients against the use of other drugs or alcoholic beverages without
first seeking their physician's advice [see Drug Interactions (7.1, 7.2)].
Inform patients that certain over-the-counter medications (e.g., antacids, cimetidine, and omeprazole), vitamins (e.g., folic acid), and herbal supplements (e.g., St. John's wort) can alter their phenytoin levels.
Hyperglycemia
Advise patients that phenytoin may cause an increase in blood glucose levels
[see Warnings and Precautions (5.14)].
Gingival Hyperplasia
Advise patients of the importance of good dental hygiene in order to minimize
the development of gingival hyperplasia and its complications.
Neurologic Effects
Counsel patients that phenytoin may cause dizziness, gait disturbance,
decreased coordination and somnolence. Advise patients taking phenytoin not to
drive, operate complex machinery, or engage in other hazardous activities
until they have become accustomed to any such effects associated with
phenytoin.
Use in Pregnancy
Inform pregnant women and women of childbearing potential that use of
phenytoin during pregnancy can cause fetal harm, including an increased risk
for cleft lip and/or cleft palate (oral clefts), cardiac defects, dysmorphic
skull and facial features, nail and digit hypoplasia, growth abnormalities
(including microcephaly), and cognitive deficits. When appropriate, counsel
pregnant women and women of childbearing potential about alternative
therapeutic options. Advise women of childbearing potential who are not
planning a pregnancy to use effective contraception while using phenytoin,
keeping in mind that there is a potential for decreased hormonal contraceptive
efficacy [see Drug Interactions (7.2)].
Instruct patients to notify their physician if they become pregnant or intend to become pregnant during therapy, and to notify their physician if they are breastfeeding or intend to breastfeed during therapy [see Use in Specific Populations (8.1, 8.2)].
Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy [see Use in Specific Populations (8.1)].
Dispense with Medication Guide
To order more Medication Guides call American Health Packaging at
1-800-707-4621.
SPL MEDGUIDE SECTION
MEDICATION GUIDE
Dispense with Medication Guide
To order more Medication Guides call American Health Packaging at
1-800-707-4621.
8427566/0825F
|
Phenytoin (fen’ i toin) Oral Suspension | |||
|
What is the most important information I should know about phenytoin oral suspension? 1. Do not stop taking phenytoin oral suspension without first talking to your healthcare provider.
2. Like other antiepileptic drugs, phenytoin oral suspension may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you: | |||
|
|
|
|
|
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. How can I watch for early symptoms of suicidal thoughts and actions?
Call your healthcare provider between visits as needed, especially if you are worried about symptoms. 3. Phenytoin oral suspension can cause a type of serious allergic reaction that may affect different parts of the body such as your liver, kidneys, blood, heart, skin or other parts of your body. These can be very serious and cause death. Call your healthcare provider right away if you have any or all of these symptoms: | |||
|
|
| |
|
Call your healthcare provider even if the symptoms are mild or if you have been taking phenytoin for an extended period of time. These symptoms can be a sign of a serious allergic reaction. 4. Phenytoin oral suspension can cause problems with your heart, including a slow heartbeat. Let your healthcare provider know right away if you have any of these symptoms: | |||
|
| ||
|
What is phenytoin oral suspension? Phenytoin oral suspension is a prescription medicine used to treat certain types of seizures called tonic-clonic (grand mal) and psychomotor (temporal lobe) seizures. | |||
|
Do not take phenytoin oral suspension if you:
| |||
|
Before taking phenytoin oral suspension, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. These medicines can change the levels of phenytoin in your blood. Taking phenytoin oral suspension with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine. | |||
|
How should I take phenytoin oral suspension?
| |||
|
What should I avoid while taking phenytoin oral suspension?
| |||
|
What are the possible side effects of phenytoin oral suspension? See**"What is the most important information I should know about phenytoin oral suspension?"** Phenytoin oral suspension may cause other serious side effects including:
Call your healthcare provider right away, if you have any of the symptoms
listed above. | |||
|
|
| |
|
Phenytoin can cause overgrowth of your gums. Brushing and flossing your teeth and seeing a dentist regularly while taking phenytoin oral suspension can help prevent this from happening. These are not all of the possible side effects of phenytoin oral suspension. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
|
How should I store phenytoin oral suspension?
Keep phenytoin oral suspension and all medicines out of the reach of children. | |||
|
General information about the safe and effective use of phenytoin oral suspension. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use phenytoin oral suspension for a condition for which it was not prescribed. Do not give phenytoin oral suspension to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about phenytoin oral suspension that is written for health professionals. | |||
|
What are the ingredients in phenytoin oral suspension? Trademarks are the property of their respective owners. For more information about phenytoin oral suspension, visit www.taro.com or call 1-866-923-4914. For more information about the packaging or labeling, call American Health Packaging at 1-800-707-4621. |
This Medication Guide has been approved by the U.S. Food and Drug Administration
Distributed by:
American Health Packaging
Columbus, OH 43217
8427566/0825F
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
FOR ORAL ADMINISTRATION ONLY; NOT FOR PARENTERAL USE
A calibrated measuring device is recommended to measure and deliver the prescribed dose accurately. A household teaspoon or tablespoon is not an adequate measuring device.
2.2 Adult Dosage
The recommended starting dosage for adult patients who have received no previous treatment is 5 mL (125 mg/5 mL), or one teaspoonful, by mouth three times daily. Adjust the dosage to suit individual requirements, up to a maximum of 25 mL daily [see Dosage and Administration (2.4)].
2.3 Pediatric Dosage
The recommended starting dosage for pediatric patients is 5 mg/kg/day by mouth in two or three equally divided doses, with subsequent dosage individualized to a maximum of 300 mg daily in divided doses. A recommended daily maintenance dosage is usually 4 mg/kg/day to 8 mg/kg/day in equally divided doses. Children over 6 years and adolescents may require the minimum adult dosage (300 mg/day).
2.4 Dosage Adjustments
Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations may be necessary for optimal dosage adjustments. Trough levels provide information about clinically effective serum level range and confirm patient compliance, and are obtained just prior to the patient's next scheduled dose. Peak levels indicate an individual's threshold for emergence of dose-related side effects and are obtained at the time of expected peak concentration. Therapeutic effect without clinical signs of toxicity occurs more often with serum total concentrations between 10 mcg/mL and 20 mcg/mL (unbound phenytoin concentrations of 1 mcg/mL to 2 mcg/mL), although some mild cases of tonic-clonic (grand mal) epilepsy may be controlled with lower serum levels of phenytoin. In patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of unbound phenytoin concentrations may be more relevant [see Dosage and Administration (2.6)].
With recommended dosages, a period of seven to ten days may be required to achieve phenytoin steady-state blood levels, and changes in dosage (increase or decrease) should not be carried out at intervals shorter than seven to ten days.
2.5 Switching Between Phenytoin Formulations
The free acid form of phenytoin is used in phenytoin oral suspension and DILANTIN Infatabs. DILANTIN extended capsules and parenteral DILANTIN are formulated with the sodium salt of phenytoin. Because there is approximately an 8% increase in drug content with the free acid form over that of the sodium salt, dosage adjustments and serum level monitoring may be necessary when switching from a product formulated with the free acid to a product formulated with the sodium salt and vice versa.
2.6 Dosing in Patients with Renal or Hepatic Impairment or Hypoalbuminemia
Because the fraction of unbound phenytoin is increased in patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of phenytoin serum levels should be based on the unbound fraction in those patients [see Warnings and Precautions (5.11) and Use in Specific Populations (8.6)].
2.7 Geriatric Dosage
Phenytoin clearance is decreased slightly in elderly patients and lower or less frequent dosing may be required [see Clinical Pharmacology (12.3)].
2.8 Dosing during Pregnancy
Decreased serum concentrations of phenytoin may occur during pregnancy because of altered phenytoin pharmacokinetics. Periodic measurement of serum phenytoin concentrations should be performed during pregnancy, and the phenytoin oral suspension dosage should be adjusted as necessary. Postpartum restoration of the original dosage will probably be indicated [see Use in Specific Populations (8.1)]. Because of potential changes in protein binding during pregnancy, the monitoring of phenytoin serum levels should be based on the unbound fraction.
- Adult starting dose in patients who have received no previous treatment is 5 mL three times daily, with dose adjustments as necessary, up to 25 mL daily. (2.2)
- Pediatric starting dose is 5 mg/kg/day in two to three equally divided doses, with dosage adjustments as necessary, up to a maximum of 300 mg daily. Maintenance dosage is 4 mg/kg/day to 8 mg/kg/day. (2.3)
- Serum blood level determinations may be necessary for optimal dosage adjustments – the clinically effective serum total concentration is 10 mcg/mL to 20 mcg/mL (unbound phenytoin concentration is 1 mcg/mL to 2 mcg/mL). (2.1)
DESCRIPTION SECTION
11 DESCRIPTION
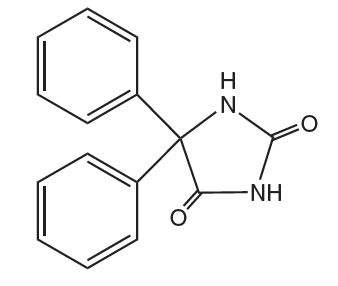
Phenytoin is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is 5,5-diphenyl-2,4 imidazolidinedione, having the following structural formula:
Each 5 mL of the oral suspension contains 125 mg of phenytoin, USP; carboxymethylcellulose sodium, citric acid anhydrous, FD&C yellow no. 6, magnesium aluminum silicate, orange flavor spray dry natural and artificial, polysorbate 60, purified water, sodium benzoate, sucrose and vanilla flavored powder artificial.
SPL UNCLASSIFIED SECTION
PACKAGING INFORMATION
American Health Packaging unit dose cups (see How Supplied section) contain
drug product from Taro Pharmaceuticals U.S.A., Inc. as follows:
(100 mg per 4 mL / 50 UD) NDC 60687-275-66 packaged from NDC 51672-4069
Distributed by:
American Health Packaging
Columbus, OH 43217
8427566/0825F
