NOREPINEPHRINE BITARTRATE IN SODIUM CHLORIDE
These highlights do not include all the information needed to use NOREPINEPHRINE BITARTRATE IN SODIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for NOREPINEPHRINE BITARTRATE IN SODIUM CHLORIDE INJECTION. NOREPINEPHRINE BITARTRATE IN SODIUM CHLORIDE injection, for intravenous use Initial U.S. Approval: 1950
ddbef60d-c20a-4ada-928f-e87074c08a54
HUMAN PRESCRIPTION DRUG LABEL
Jul 31, 2023
WG Critical Care, LLC
DUNS: 829274633
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
NOREPINEPHRINE BITARTRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
NOREPINEPHRINE BITARTRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
NOREPINEPHRINE BITARTRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel

NDC 44567-642-01
Norepinephrine Bitartrate in 0.9% Sodium Chloride Injection
16 mg per 250 mL (64 mcg per mL)
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Norepinephrine Bitartrate in Sodium Chloride Injection is indicated to raise blood pressure in adult patients with severe, acute hypotension.
Norepinephrine Bitartrate in Sodium Chloride Injection is a catecholamine indicated for restoration of blood pressure in adult patients with acute hypotensive states. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
None. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Tissue Ischemia
Administration of Norepinephrine Bitartrate in Sodium Chloride Injection to patients who are hypotensive from hypovolemia can result in severe peripheral and visceral vasoconstriction, decreased renal perfusion and reduced urine output, tissue hypoxia, lactic acidosis, and reduced systemic blood flow despite "normal" blood pressure. Address hypovolemia prior to initiating Norepinephrine Bitartrate in Sodium Chloride Injection [see Dosage and Administration (2.1)]. Avoid Norepinephrine Bitartrate in Sodium Chloride Injection in patients with mesenteric or peripheral vascular thrombosis, as this may increase ischemia and extend the area of infarction.
Gangrene of the extremities has occurred in patients with occlusive or thrombotic vascular disease or who received prolonged or high dose infusions. Monitor for changes to the skin of the extremities in susceptible patients.
Extravasation of Norepinephrine Bitartrate in Sodium Chloride Injection may cause necrosis and sloughing of surrounding tissue. To reduce the risk of extravasation, infuse into a large vein, check the infusion site frequently for free flow, and monitor for signs of extravasation [see Dosage and Administration (2.1)].
Emergency Treatment of Extravasation
To prevent sloughing and necrosis in areas in which extravasation has occurred, infiltrate the ischemic area as soon as possible, using a syringe with a fine hypodermic needle with 5 to 10 mg of phentolamine mesylate in 10 to 15 mL of 0.9% Sodium Chloride Injection in adults.
Sympathetic blockade with phentolamine causes immediate and conspicuous local hyperemic changes if the area is infiltrated within 12 hours.
5.2 Hypotension after Abrupt Discontinuation
Sudden cessation of the infusion rate may result in marked hypotension. When discontinuing the infusion, gradually reduce the Norepinephrine Bitartrate in Sodium Chloride Injection infusion rate while expanding blood volume with intravenous fluids.
5.3 Cardiac Arrhythmias
Norepinephrine Bitartrate in Sodium Chloride Injection elevates intracellular calcium concentrations and may cause arrhythmias, particularly in the setting of hypoxia or hypercarbia. Perform continuous cardiac monitoring of patients with arrhythmias.
•
Tissue Ischemia: Avoid extravasation into tissues, which can cause local necrosis. (5.1)
•
Hypotension After Abrupt Discontinuation: Sudden cessation of the infusion rate may result in marked hypotension. Reduce the Norepinephrine Bitartrate in Sodium Chloride Injection infusion rate gradually. (5.2)
•
Cardiac Arrhythmias: Norepinephrine Bitartrate in Sodium Chloride Injection may cause arrhythmias. Monitor cardiac function in patients with underlying heart disease. (5.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
•
Tissue Ischemia [see Warnings and Precautions (5.1)]
•
Hypotension [see Warnings and Precautions (5.2)]
•
Cardiac Arrhythmias [see Warnings and Precautions (5.3)]
The most common adverse reactions are hypertension and bradycardia.
The following adverse reactions can occur:
Nervous system disorders: Anxiety, headache
Respiratory disorders: Respiratory difficulty, pulmonary edema
Most common adverse reactions are ischemic injury, bradycardia, anxiety, transient headache, respiratory difficulty, and extravasation necrosis at injection site. (6)
To report SUSPECTED ADVERSE REACTIONS, contact WG Critical Care, LLC at 1-866-562-4708, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 MAO-Inhibiting Drugs
Co-administration of Norepinephrine Bitartrate in Sodium Chloride Injection with monoamine oxidase (MAO) inhibitors or other drugs with MAO-inhibiting properties (e.g., linezolid) can cause severe, prolonged hypertension.
If administration of Norepinephrine Bitartrate in Sodium Chloride Injection cannot be avoided in patients who recently have received any of these drugs and in whom, after discontinuation, MAO activity has not yet sufficiently recovered, monitor for hypertension.
7.2 Tricyclic Antidepressants
Co-administration of Norepinephrine Bitartrate in Sodium Chloride Injection with tricyclic antidepressants (including amitriptyline, nortriptyline, protriptyline, clomipramine, desipramine, imipramine) can cause severe, prolonged hypertension. If administration of Norepinephrine Bitartrate in Sodium Chloride Injection cannot be avoided in these patients, monitor for hypertension.
7.3 Antidiabetics
Norepinephrine Bitartrate in Sodium Chloride Injection can decrease insulin sensitivity and raise blood glucose. Monitor glucose and consider dosage adjustment of antidiabetic drugs.
7.4 Halogenated Anesthetics
Concomitant use of Norepinephrine Bitartrate in Sodium Chloride Injection with halogenated anesthetics (e.g., cyclopropane, desflurane, enflurane, isoflurane, and sevoflurane) may lead to ventricular tachycardia or ventricular fibrillation. Monitor cardiac rhythm in patients receiving concomitant halogenated anesthetics.
•
Monoamine oxidase inhibitors (MAOI) or tricyclic antidepressants of the triptyline or imipramine types may result in hypertension. (7.1, 7.2)
•
Antidiabetics: Norepinephrine can decrease insulin sensitivity and raise blood glucose. (7.3)
•
Cyclopropane and halothane anesthetics increase cardiac autonomic irritability. (7.4)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
Correct Hypovolemia
Address hypovolemia before initiation of Norepinephrine Bitartrate in Sodium Chloride Injection therapy. If the patient does not respond to therapy, suspect occult hypovolemia [see Warnings and Precautions (5.1)].
Administration
Norepinephrine Bitartrate in Sodium Chloride Injection is a ready to administer product that requires no further dilution prior to infusion. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Infuse Norepinephrine Bitartrate in Sodium Chloride Injection into a large vein. Avoid infusions into the veins of the leg in the elderly or in patients with occlusive vascular disease of the legs [see Warnings and Precautions (5.1)]. Avoid using a catheter-tie-in technique.
Discontinuation
When discontinuing the infusion, reduce the flow rate gradually. Avoid abrupt withdrawal. Discard unused portion.
2.2 Dosage
After an initial dosage of 8 to 12 mcg per minute via intravenous infusion, assess patient response and adjust dosage to maintain desired hemodynamic effect. Monitor blood pressure every two minutes until the desired hemodynamic effect is achieved, and then monitor blood pressure every five minutes for the duration of the infusion.
Typical maintenance intravenous dosage is 2 to 4 mcg per minute.
2.4 Drug Incompatibilities
Avoid contact with iron salts, alkalis, or oxidizing agents.
Whole blood or plasma, if indicated to increase blood volume, should be administered separately.
•
Initiate 8 to 12 mcg/min and adjust the rate to maintain blood pressure sufficient to maintain the circulation of vital organs. (2.2)
•
The average maintenance dose ranges from 2 to 4 mcg/min. (2.2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Injection:
Norepinephrine Bitartrate in Sodium Chloride is a clear, colorless solution, available as:
•
4 mg/250 mL (16 mcg per mL) of norepinephrine (free base), single-dose bags
•
8 mg/250 mL (32 mcg per mL) of norepinephrine (free base), single-dose bags
•
16 mg/250 mL (64 mcg per mL) of norepinephrine (free base), single-dose bags
Injection:
•
4 mg/250 mL (16 mcg per mL) of norepinephrine, single-dose bags (3)
•
8 mg/250 mL (32 mcg per mL) of norepinephrine, single-dose bags. (3)
•
16 mg/250 mL (64 mcg per mL) of norepinephrine, single-dose bags (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published data consisting of a small number of case reports and multiple small trials involving the use of norepinephrine in pregnant women at the time of delivery have not identified an increased risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There are risks to the mother and fetus from hypotension associated with septic shock, myocardial infarction and stroke which are medical emergencies in pregnancy and can be fatal if left untreated. (see Clinical Considerations). In animal reproduction studies, using high doses of intravenous norepinephrine resulted in lowered maternal placental blood flow. Clinical relevance to changes in the human fetus is unknown since the average maintenance dose is ten times lower (see Data). Increased fetal reabsorptions were observed in pregnant hamsters after receiving daily injections at approximately 2 times the maximum recommended dose on a mg/m3 basis for four days during organogenesis (see Data).
The estimated background risk for major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypotension associated with septic shock, myocardial infarction, and stroke are medical emergencies in pregnancy which can be fatal if left untreated. Delaying treatment in pregnant women with hypotension associated with septic shock, myocardial infarction and stroke may increase the risk of maternal and fetal morbidity and mortality. Life-sustaining therapy for the pregnant woman should not be withheld due to potential concerns regarding the effects of norepinephrine on the fetus.
Data
Animal Data
A study in pregnant sheep receiving high doses of intravenous norepinephrine (40 mcg/min, at approximately 10 times the average maintenance dose of 2–4 mcg/min in human, on a mg/kg basis) exhibited a significant decrease in maternal placental blood flow. Decreases in fetal oxygenation, urine and lung liquid flow were also observed.
Norepinephrine administration to pregnant rats on Gestation Day 16 or 17 resulted in cataract production in rat fetuses.
In hamsters, an increased number of resorptions (29.1% in study group vs. 3.4% in control group), fetal microscopic liver abnormalities and delayed skeletal ossification were observed at approximately 2 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day from Gestation Day 7–10).
8.2 Lactation
Risk Summary
There are no data on the presence of norepinephrine in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. Clinically relevant exposure to the infant is not expected based on the short half-life and poor oral bioavailability of norepinephrine.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of Norepinephrine Bitartrate in Sodium Chloride Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Avoid administration of Norepinephrine Bitartrate in Sodium Chloride Injection into the veins in the leg in elderly patients [see Warnings and Precautions (5.1)].
•
Elderly patients may be at greater risk of developing adverse reactions. (8.5)
OVERDOSAGE SECTION
10 OVERDOSAGE
Overdosage with Norepinephrine Bitartrate in Sodium Chloride Injection may result in headache, severe hypertension, reflex bradycardia, marked increase in peripheral resistance, and decreased cardiac output.
In case of overdosage, discontinue Norepinephrine Bitartrate in Sodium Chloride Injection until the condition of the patient stabilizes.
DESCRIPTION SECTION
11 DESCRIPTION
Norepinephrine Bitartrate in Sodium Chloride Injection contains the active pharmaceutical ingredient norepinephrine, a catecholamine, in the form of bitartrate salt (monohydrate). Norepinephrine is sometimes referred to as l-arterenol/Levarterenol or l-norepinephrine which differs from epinephrine by the absence of a methyl group on the nitrogen atom.
The chemical name for norepinephrine bitartrate (monohydrate) is (-)-α-(aminomethyl)-3,4-dihydroxybenzyl alcohol tartrate (1:1) (salt) monohydrate (molecular weight 337.3 g/mol) and has the following structural formula:
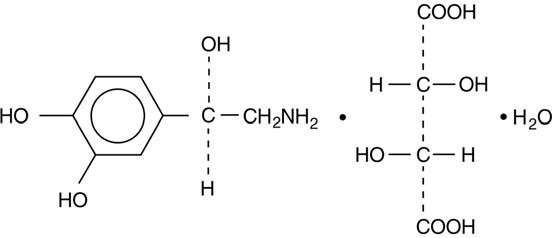
Norepinephrine bitartrate (monohydrate) is sparingly soluble in water, very slightly soluble in alcohol and ether, and readily soluble in acids.
Norepinephrine Bitartrate in Sodium Chloride Injection is supplied as a sterile aqueous ready to use solution in 250 mL transparent intravenous bags. Each mL contains 32, 64 or 128 micrograms of norepinephrine bitartrate monohydrate, equivalent to 16, 32, or 64 micrograms of norepinephrine base, respectively. Each mL also contains 9 mg of Sodium Chloride USP as tonicity agent, and may contain Hydrochloric Acid NF and Sodium Hydroxide NF as pH adjusters, for the pH range of 3.4 to 4.0, in Water for Injection.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Norepinephrine Bitartrate in Sodium Chloride Injection is filled in 250 mL transparent intravenous Nexcel bags as:
|
NDC Configuration |
Packaging Configuration |
Total Norepinephrine Bitartrate |
|
44567-640-01 |
1 single-dose bag |
4 mg per 250 mL (16 mcg per mL) |
|
44567-640-10 |
10 bags per carton | |
|
44567-641-01 |
1 single-dose bag |
8 mg per 250 mL (32 mcg per mL) |
|
44567-641-10 |
10 bags per carton | |
|
44567-642-01 |
1 single-dose bag |
16 mg per 250 mL (64 mcg per mL) |
|
44567-642-10 |
10 bags per carton |
Each filled bag is packed in an overwrap with a transparent band and oxygen absorber and oxygen indicator placed inside the overwrapping to prevent deterioration of drug product. Product should be used within 7 days of removal from overwrap.
Do not use the product if the oxygen indicator has changed color to green or blue before opening the overwrap. Normal color is yellow or orange.
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature] in the original carton to protect from light. Do not freeze.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Risk of Tissue Damage
Advise the patient, family, or caregiver to report signs of extravasation urgently [see Warnings and Precautions (5.1)].
SPL UNCLASSIFIED SECTION
Manufactured for:
WG Critical Care, LLC
Paramus, NJ 07652
Made in Switzerland
U.S. Patent Number 10,888,534
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Norepinephrine is a peripheral vasoconstrictor (alpha-adrenergic action) and an inotropic stimulator of the heart and dilator of coronary arteries (beta- adrenergic action).
12.2 Pharmacodynamics
The primary pharmacodynamic effects of norepinephrine are cardiac stimulation and vasoconstriction. Cardiac output is generally unaffected, although it can be decreased, and total peripheral resistance is also elevated. The elevation in resistance and pressure result in reflex vagal activity, which slows the heart rate and increases stroke volume. The elevation in vascular tone or resistance reduces blood flow to the major abdominal organs as well as to skeletal muscle. Coronary blood flow is substantially increased secondary to the indirect effects of alpha stimulation. After intravenous administration, a pressor response occurs rapidly and reaches steady state within 5 minutes. The pharmacologic actions of norepinephrine are terminated primarily by uptake and metabolism in sympathetic nerve endings. The pressor action stops within 1–2 minutes after the infusion is discontinued.
12.3 Pharmacokinetics
Absorption
Following initiation of intravenous infusion, the steady state plasma concentration is achieved in 5 min.
Distribution
Plasma protein binding of norepinephrine is approximately 25%. It is mainly bound to plasma albumin and to a smaller extent to prealbumin and alpha 1-acid glycoprotein. The volume of distribution is 8.8 L. Norepinephrine localizes mainly in sympathetic nervous tissue. It crosses the placenta but not the blood-brain barrier.
Elimination
The mean half-life of norepinephrine is approximately 2.4 min. The average metabolic clearance is 3.1 L/min.
Metabolism
Norepinephrine is metabolized in the liver and other tissues by a combination of reactions involving the enzymes catechol-O-methyltransferase (COMT) and MAO. The major metabolites are normetanephrine and 3-methoxyl-4-hydroxy mandelic acid (vanillylmandelic acid, VMA), both of which are inactive. Other inactive metabolites include 3-methoxy-4-hydroxyphenylglycol, 3,4-dihydroxymandelic acid, and 3,4-dihydroxyphenylglycol.
Excretion
Noradrenaline metabolites are excreted in urine primarily as sulphate conjugates and, to a lesser extent, as glucuronide conjugates. Only small quantities of norepinephrine are excreted unchanged.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis, mutagenesis, and fertility studies have not been performed.
