Morphine Sulfate
These highlights do not include all the information needed to use MORPHINE SULFATE INJECTION safely and effectively. See full prescribing information for MORPHINE SULFATE INJECTION. MORPHINE SULFATE INJECTION, PRESERVATIVE-FREE, Solution for Intravenous Use, CII Initial U.S. Approval: 1941
be420e8b-bcb0-49b5-bb4d-1df8b9959809
HUMAN PRESCRIPTION DRUG LABEL
Jul 30, 2025
Hospira, Inc.
DUNS: 141588017
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
MORPHINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
MORPHINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
MORPHINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
MORPHINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
MORPHINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 mg/mL Syringe Luer Lock Cello Pack Label
NDC 0409-1893-23
Preservative-Free
Morphine Sulfate
Injection, USP
CII
10 mg / mL
INTRAVENOUS USE ONLY
Rx only
10 NexJect 1 mL Single-dose syringes with luer lock
Needle not included
Protect from light and freezing. Opaque covering needed until contents
are used.
Store at 20 to 25°C (68 to 77°F). (See USP Controlled Room Temperature.)
Sterile Aqueous Injection
Usual Dosage: See Package Insert.
Each mL contains morphine sulfate 10 mg, edetate disodium 0.2 mg, citric acid
0.4 mg, and sodium chloride 7.8 mg. Hydrochloric acid and/or sodium hydroxide
may
be added to adjust pH to 3.0 (2.5 to 4.0).
The injection is not to be used if its color is darker than pale yellow, if it
is discolored
in any other way, or if it contains a precipitate.

DESCRIPTION SECTION
11 DESCRIPTION
Morphine Sulfate Injection, USP is an opioid agonist, available in 2 mg/mL, 4 mg/mL, 8 mg/mL, 10 mg/mL, and 15 mg/mL (1 mL fill in 2.5 mL Carpuject™ Single‑dose cartridge with Luer Lock for the Carpuject™ Syringe System) and 2 mg/mL, 4 mg/mL, 8 mg/mL, and 10 mg/mL (1 mL fill in 1.5 mL NexJect™ Single- dose Prefilled Syringe with Luer Lock). When exposed to air it gradually loses water of hydration, and darkens on prolonged exposure to light. The chemical name is 7,8-Didehydro-4,5-epoxy-17-methyl-(5α,6α)-morphinan-3,6-diol sulfate (2: 1) (salt), pentahydrate, with the following chemical structure:
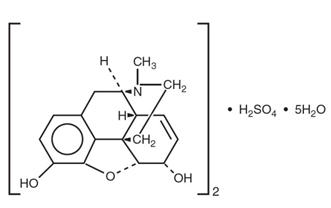
(C17H19NO3)2 . H2SO4 . 5H2O Molecular Weight is 758.83
Morphine sulfate USP is an odorless, white crystalline powder with a bitter taste. It has a solubility of 1 in 21 parts of water and 1 in 1000 parts of alcohol, but is practically insoluble in chloroform or ether. The octanol:water partition coefficient of morphine is 1.42 at physiologic pH and the pKa is 7.9 for the tertiary nitrogen (the majority is ionized at pH 7.4).
Morphine Sulfate Injection, USP is a sterile, nonpyrogenic solution of morphine sulfate, free of antioxidants and preservatives to be administered by the intravenous route.
For the single-dose Carpuject™ cartridges for intravenous administration:
Each milliliter of sterile solution contains 2 mg, 4 mg, 8 mg, 10 mg, or 15 mg Morphine Sulfate Injection, USP and the following inactive ingredients: 0.2 mg edetate disodium, 0.4 mg citric acid for the 2 mg, 4 mg, 8 mg and 10 mg Morphine Sulfate Injection, USP or 0.8 mg citric acid for the 15 mg Morphine Sulfate Injection, USP, sodium chloride to adjust isotonicity and water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH. The pH range is 2.5 to 4.0.
For the single-dose NexJect™ syringes for intravenous administration: Each milliliter of sterile solution contains 2 mg, 4 mg, 8 mg, or 10 mg Morphine Sulfate Injection, USP and the following inactive ingredients: 0.2 mg edetate disodium, 0.4 mg citric acid for the 2 mg, 4 mg, 8 mg, and 10 mg Morphine Sulfate Injection, USP, sodium chloride to adjust isotonicity and water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH. The pH range is 2.5 to 4.0.
SPL UNCLASSIFIED SECTION
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA

LAB-0841-6.0
INSTRUCTIONS FOR USE SECTION
INSTRUCTIONS FOR USE
Instructions for use - Carpuject™ Single-dose Cartridge
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if color is darker than pale yellow, if it is discolored in any other way, or if it contains a precipitate.
Instructions for use - Carpuject™ Single-dose Cartridge
Carpuject™ Single-dose cartridges with Luer Lock are packaged in a Slim-Pak™ tamper detection package. Note that a needle is not included.
Before use, read all instructions for using the Carpuject™ Syringe, which are contained in the product insert for the reusable Carpuject™ Holder before use.
Carpuject™ Single-dose cartridges are to be used ONLY with Carpuject™ Holders.
NOTE: To prevent needlestick injuries, do not recap, purposely bend, or break by hand used needles. Do not recap, purposely bend, or break by hand blunt Cannulas.
Instructions for use - NexJect™ Single-dose Prefilled Syringe
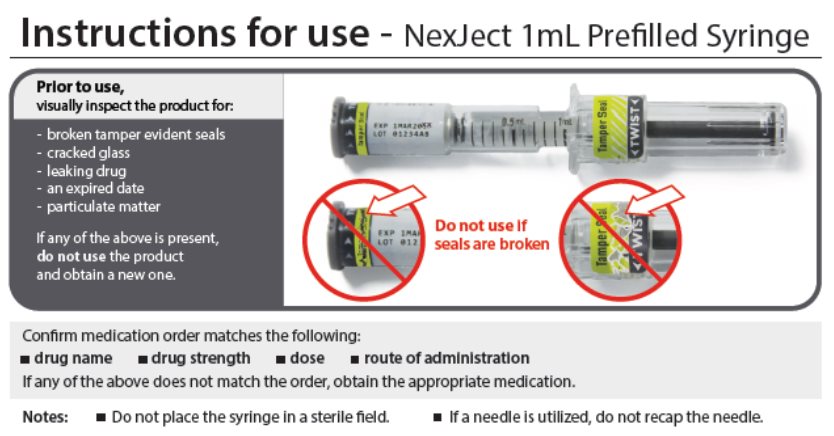
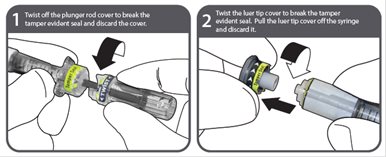
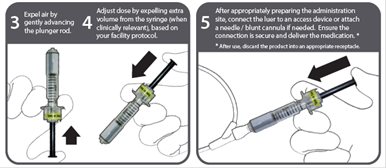
LAB-0921-4.0
Revised: 12/2023
