acyclovir
Acyclovir Capsules, USP Rx only
c818e86c-a4d0-48cd-a888-fa1ee9e2aa19
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
acyclovir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
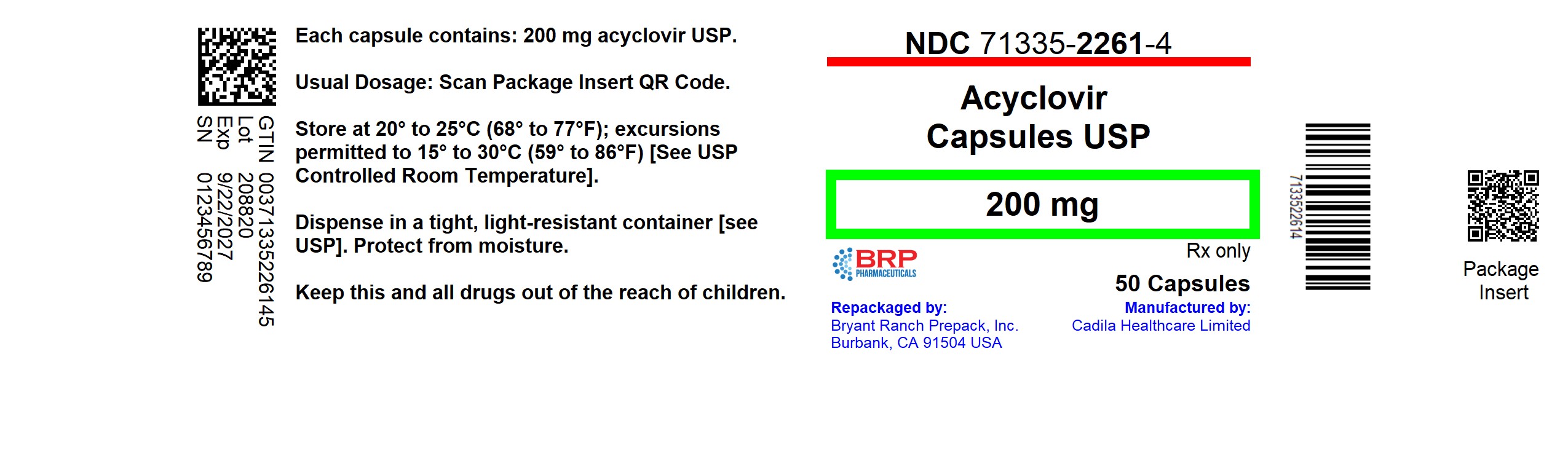
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Acyclovir 200mg Capsule
DESCRIPTION SECTION
DESCRIPTION
Acyclovir is a synthetic nucleoside analogue active against herpesviruses. Acyclovir capsules are formulation for oral administration.
Acyclovir is a white or almost white, crystalline powder with the molecular formula C8H11N5O3 and a molecular weight of 225.20. It is soluble in diluted hydrochloric acid; slightly soluble in water and insoluble in alcohol. The pka’s of acyclovir are 2.27 and 9.25.
The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:

Each acyclovir capsule intended for oral administration contains 200 mg of acyclovir. In addition, each capsule contains the following inactive ingredients: corn starch, FD & C blue # 1, FD & C red # 3, gelatin, lactose monohydrate, magnesium stearate, sodium lauryl sulfate and titanium dioxide. Each capsule is printed with black pharmaceutical ink which contains black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
HOW SUPPLIED SECTION
HOW SUPPLIED
Acyclovir Capsules USP, 200 mg are white to off-white powder filled in size "1" empty hard gelatin capsules with blue opaque colored cap imprinted with '668' in black ink and white opaque colored body and are supplied as follows:
NDC: 71335-2261-1: 30 Capsules in a BOTTLE
NDC: 71335-2261-2: 25 Capsules in a BOTTLE
NDC: 71335-2261-3: 35 Capsules in a BOTTLE
NDC: 71335-2261-4: 50 Capsules in a BOTTLE
NDC: 71335-2261-5: 100 Capsules in a BOTTLE
NDC: 71335-2261-6: 40 Capsules in a BOTTLE
NDC: 71335-2261-7: 60 Capsules in a BOTTLE
NDC: 71335-2261-8: 120 Capsules in a BOTTLE
NDC: 71335-2261-9: 20 Capsules in a BOTTLE
NDC: 71335-2261-0: 90 Capsules in a BOTTLE
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
