Acyclovir
Acyclovir Tablets USP
b24201ea-aeae-4de1-b01b-eee89068e786
HUMAN PRESCRIPTION DRUG LABEL
Jul 2, 2023
SQUARE PHARMACEUTICALS LIMITED
DUNS: 731487153
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Acyclovir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Acyclovir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
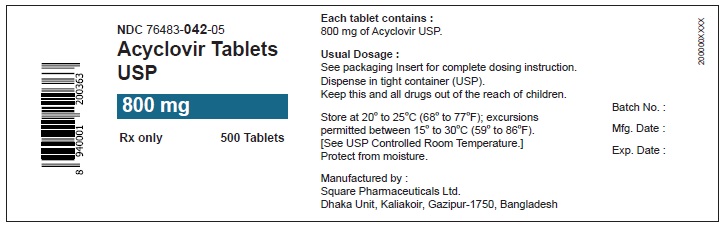
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package Label – 800mg
Rx Only
NDC 76483-042-05
Acyclovir Tablets USP
800mg
500 Tablets
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F).
[See USP Controlled Room Temperature.]
Protect from moisture.
Dispense in a tight container as defined in the USP.
See prescribing information for dosage information.
PHARMACODYNAMICS SECTION
VIROLOGY
Mechanism of Antiviral Action:
Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV).
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir mono phosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared with VZV is due to its more efficient phosphorylation by the viral TK.
Antiviral Activities:
The quantitative relationship between the in vitro susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50 % the growth of virus in cell culture (IC50), vary greatly depending upon a number of factors. Using plaque-reduction assays, the IC50 against herpes simplex virus isolates ranges from 0.02 to 13.5mcg/mL for HSV-1 and from 0.01 to 9.9mcg/mL for HSV-2. The IC50 for acyclovir against most laboratory strains and clinical isolates of VZV ranges from 0.12 to 10.8mcg/mL. Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean IC50 of 1.35mcg/mL.
Drug Resistance:
Resistance of HSV and VZV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV and VZV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir-resistant mutants isolated thus far from immunocompromised patients have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Pharmacokinetics:
The pharmacokinetics of acyclovir after oral administration have been evaluated in healthy volunteers and in immunocompromised patients with herpes simplex or varicella-zoster virus infection. Acyclovir pharmacokinetic parameters are summarized in Table 1.
Table 1. Acyclovir Pharmacokinetic Characteristics (Range)|
Parameter |
Range |
|
Plasma protein binding |
9 % to 33% |
|
Plasma elimination half-life |
2.5 to 3.3 hr |
|
Average oral bio availability |
10 % to 20 %* |
|
Bio availability decreases with increasing dose.*** |
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Acute Treatment of Herpes Zoster:
800mg every 4 hours orally, 5 times daily for 7 to 10 days.
Genital Herpes:
Treatment of Initial Genital Herpes:
200mg every 4 hours, 5 times daily for 10 days.
Chronic Suppressive Therapy for Recurrent Disease:
400mg 2 times daily for up to 12 months, followed by re-evaluation. Alternative regimens have included doses ranging from 200mg 3 times daily to 200mg 5 times daily.
The frequency and severity of episodes of untreated genital herpes may change over time. After 1 year of therapy, the frequency and severity of the patient's genital herpes infection should be re-evaluated to assess the need for continuation of therapy with acyclovir.
Intermittent Therapy:
200mg every 4 hours, 5 times daily for 5 days. Therapy should be initiated at the earliest sign or symptom (prodrome) of recurrence.
Treatment of Chickenpox:
Children (2 years of age and older):
20mg/kg per dose orally 4 times daily (80mg/kg/day) for 5 days. Children over 40kg should receive the adult dose for chickenpox.
Adults and Children over 40 kg:
800mg 4 times daily for 5 days.
Intravenous acyclovir is indicated for the treatment of varicella-zoster infections in immunocompromised patients.
When therapy is indicated, it should be initiated at the earliest sign or symptom of chickenpox. There is no information about the efficacy of therapy initiated more than 24 hours after onset of signs and symptoms.
Patients With Acute or Chronic Renal Impairment:
In patients with renal impairment, the dose of acyclovir Tablets should be modified as shown in Table 3.
** Table 3. Dosage Modification for Renal Impairment**
|
Normal Dosage Regimen |
Creatinine Clearance (mL/min/1.73 m2) |
Adjusted Dosage Regimen | |
|
Dose (mg) |
Dosing Interval | ||
|
200 mg every 4 hours |
|
200 |
every 4 hours, 5x daily |
|
400 mg every 12 hours |
|
400 |
every 12 hours |
|
800 mg every 4 hours |
|
800 |
every 4 hours, 5x daily |
Hemodialysis:
For patients who require hemodialysis, the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. This results in a 60 % decrease in plasma concentrations following a 6-hour dialysis period. Therefore, the patient's dosing schedule should be adjusted so that an additional dose is administered after each dialysis.
Peritoneal Dialysis:
No supplemental dose appears to be necessary after adjustment of the dosing interval.
