Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
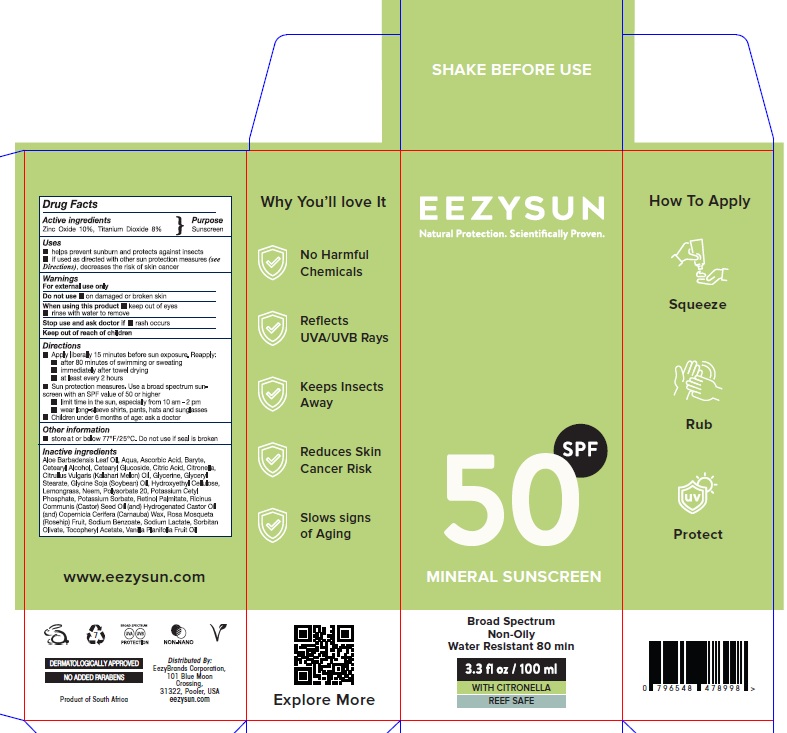
Eezysun SPF 50
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Product label

INDICATIONS & USAGE SECTION
Uses
Helps prevent sunburn and protects against insects
If used as directed with other sun protection measures (See Directions)
decreases the risk of skin cancer
DOSAGE & ADMINISTRATION SECTION
Direction
- Apply liberally 15 minutes before sun exposure. Reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun protection measures. Use a broad spectrum sun-screen with an SPF value of 50 or higher
- limit time in the sun, especially from 10 am - 2 pm
- wear long-sleeve shirts, pants, hats and sunglasses
- Children under 6 months of age: ask a doctor
Shake well before use
WARNINGS SECTION
Warnings
*For external use only *Do not useon damaged or broken skin *When using this productkeep out of eyes ,rinse with water to remove
*Stop use and ask doctor ifrash occurs *Keep out of reach of children
OTC - ACTIVE INGREDIENT SECTION
Active ingredients
Zinc Oxide 10%, Titanium Dioxide 8%
OTC - PURPOSE SECTION
Purpose
Sunscreen
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
SPL UNCLASSIFIED SECTION
Other information
store at or below 77°F/25°C . Do not use if seal is broken
INACTIVE INGREDIENT SECTION
Inactive ingredients
Aloe Barbadensis Leaf Oil, Aqua, Ascorbic Acid, Baryte,Cetearyl Alcohol,
Cetearyl Glucoside, Citric Acid, Citronella,Citrullus Vulgaris (Kalahari
Melon) Oil, Glycerine, Glyceryl Stearate, Glycine Soja (Soybean) Oil,
Hydroxyethyl Cellulose,Lemongrass, Neem, Polysorbate 20, Potassium Cetyl
Phosphate, Potassium Sorbate, Retinol Palmitate, Ricinus Communis (Castor)
Seed Oil (and) Hydrogenated Castor Oil (and) Copernicia Cerifera (Carnauba)
Wax, Rosa Mosqueta (Rosehip) Fruit, Sodium Benzoate, Sodium Lactate, Sorbitan
Olivate, Tocopheryl Acetate, Vanilla Planifolia Fruit Oil
