OneLAX SENNA
OneLAX SENNA SYRUP Natural Vegitable Lexative Natural Choclate Flavor
d2a5a13a-e7e4-488a-b60b-4dd467efa971
HUMAN OTC DRUG LABEL
May 23, 2025
ATLANTIC BIOLOGICALS CORP.
DUNS: 047437707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SENNA
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


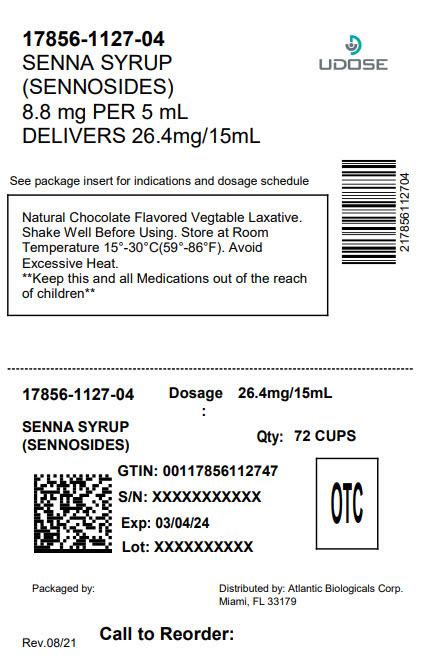
INDICATIONS & USAGE SECTION
Directions
take preferably at bedtime or as directed by a doctor. Shake Well Before Using.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Active ingredient(in each teaspoonful)Purpose
Sennosides 8.8 mg …………………………................Laxative
OTC - PURPOSE SECTION
Uses
relieves occasional constipation (irregularity), generally causes bowel movement in 6 to 12 hours
WARNINGS SECTION
Warnings
ask a doctor before use if you have • stomach pain • nausea • vomiting • noticed a sudden change in bowel movements that continues over a period of 2 weeks
Do not use laxative products for longer than 1 week unless directed by a doctor
Ask a doctor or pharmacist before use if
you are taking any other drug. Take this product two more hours before or after other drugs. Laxatives may affect how other drugs work.
Stop use and ask a doctor if
you have rectal bleeding or fail to have bowel movement after use of laxative. This may indicate a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
|
** age** |
** starting dose** |
** maximum dosage** |
|
Adults and children 12 yeart and older |
2-3 teaspoons once a day |
3 teaspoons twice a day |
|
Children 6 years to under 12 yeart |
1 - 1 1/2teaspoons once a day |
1 1/2teaspoons twice a day |
|
Children 2 years to under 6 yeart |
l/2- 3/4teaspoon once a day |
3/4 teaspoon twice a day |
|
Children under 2 years |
ask a doctor |
ask a doctor |
STORAGE AND HANDLING SECTION
Other Information
- Avoid accessive heat. Store at room temperature 15°-30°C (59°-86°F)
INACTIVE INGREDIENT SECTION
Inactive Ingredients
flavor choclate, mythylparaben, propylene glycol, propylparaben, purified water and sucrose
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
KEEP OUT OF REACH OF CHILDREN SECTION
In case of accidental overdose, get medical help or contact a Poison Control Center immediately
Questions?
To Report Adverse Drug Event Call 1(877) 225-6999
DISTRIBUTED BY:
ATLANTIC BIOLOGICALS CORP.
MIAMI, FL 33179
