Doxylamine Succinate and Pyridoxine Hydrochloride
These highlights do not include all the information needed to use DOXYLAMINE SUCCINATE AND PYRIDOXINE HYDROCHLORIDE DELAYED-RELEASE TABLETS safely and effectively. See full prescribing information for DOXYLAMINE SUCCINATE AND PYRIDOXINE HYDROCHLORIDE DELAYED-RELEASE TABLETS. DOXYLAMINE SUCCINATE and PYRIDOXINE HYDROCHLORIDE delayed-release tablets, for oral useInitial U.S. Approval: 1976
957865a2-efcf-43a6-92a8-f9f980892833
HUMAN PRESCRIPTION DRUG LABEL
Nov 30, 2022
Mylan Pharmaceuticals Inc.
DUNS: 059295980
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
doxylamine succinate and pyridoxine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – 10 mg
NDC 0378-4615-01
Doxylamine
Succinate and
Pyridoxine HCl
Delayed-Release
Tablets
10 mg/10 mg
WARNING: Swallow tablets whole.
Do not crush, chew, or split the tablets.
Rx only 100 Tablets
Each film-coated tablet contains:
Doxylamine succinate, USP 10 mg
Pyrixodine hydrochloride, USP 10 mg
Usual Dosage: Take two tablets daily at
bedtime. If symptoms are not adequately
controlled, the dose can be increased to a
maximum recommended dose of four tablets
daily (one in the morning, one mid-afternoon
and two at bedtime). See accompanying
prescribing information.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from moisture and light.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Made in India
Mylan.com
RMX4615A
Keep this and all medication out of the
reach of children.
Dispense in original container or equivalent
air tight, light resistant container.
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Activities Requiring Mental Alertness
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets may cause somnolence due to the anticholinergic properties of doxylamine succinate, an antihistamine. Women should avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using doxylamine succinate and pyridoxine hydrochloride delayed-release tablets until cleared to do so by their healthcare provider.
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets use is not recommended if a woman is concurrently using central nervous system (CNS) depressants including alcohol. The combination may result in severe drowsiness leading to falls or accidents [see Drug Interactions (7.1)].
5.2 Concomitant Medical Conditions
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets have anticholinergic properties and, therefore, should be used with caution in women with: increased intraocular pressure, narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction and urinary bladder-neck obstruction.
5.3 Interference with Urine Screen for Methadone, Opiates and Phencyclidine
Phosphate (PCP)
There have been reports of false positive urine screening tests for methadone, opiates, and PCP with doxylamine succinate/pyridoxine hydrochloride use [see Drug Interactions (7.3)].
•
Activities requiring mental alertness: Avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using doxylamine succinate and pyridoxine hydrochloride delayed-release tablets until cleared to do so by a healthcare provider. (5.1)
•
Central nervous system (CNS) depressants: Concurrent use with alcohol or other CNS depressants is not recommended. (5.1)
•
Anticholinergic actions: Use with caution in patients with increased intraocular pressure, narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction and urinary bladder-neck obstruction. (5.2)
•
Interference with urine drug screen: Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets may interfere with urine screening for methadone, opiates and PCP. (5.3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
Initially, take two doxylamine succinate and pyridoxine hydrochloride delayed- release tablets orally at bedtime (Day 1). If this dose adequately controls symptoms the next day, continue taking two tablets daily at bedtime. However, if symptoms persist into the afternoon of Day 2, take the usual dose of two tablets at bedtime that night then take three tablets starting on Day 3 (one tablet in the morning and two tablets at bedtime). If these three tablets adequately control symptoms on Day 4, continue taking three tablets daily. Otherwise take four tablets starting on Day 4 (one tablet in the morning, one tablet mid-afternoon and two tablets at bedtime).
The maximum recommended dose is four tablets (one in the morning, one in the mid-afternoon and two at bedtime) daily.
Take on an empty stomach with a glass of water [see Clinical Pharmacology (12.3)]. Swallow tablets whole. Do not crush, chew, or split doxylamine succinate and pyridoxine hydrochloride delayed-release tablets.
Take as a daily prescription and not on an as needed basis. Reassess the woman for continued need for doxylamine succinate and pyridoxine hydrochloride delayed-release tablets as her pregnancy progresses.
Take two tablets daily at bedtime. If symptoms are not adequately controlled, the dose can be increased to a maximum recommended dose of four tablets daily (one in the morning, one mid-afternoon and two at bedtime) as described in the full prescribing information. (2)
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdose
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets are a delayed-release formulation, therefore, signs and symptoms of intoxication may not be apparent immediately.
Signs and symptoms of overdose may include restlessness, dryness of mouth, dilated pupils, sleepiness, vertigo, mental confusion and tachycardia.
At toxic doses, doxylamine exhibits anticholinergic effects, including seizures, rhabdomyolysis, acute renal failure and death.
10.2 Management of Overdose
If treatment is needed, it consists of gastric lavage or activated charcoal, whole bowel irrigation and symptomatic treatment. For additional information about overdose treatment, call a poison control center (1-800-222-1222).
DESCRIPTION SECTION
11 DESCRIPTION
Doxylamine succinate and pyridoxine hydrochloride delayed-release tablets are round, white to off-white, film-coated, delayed-release tablets containing 10 mg of doxylamine succinate and 10 mg of pyridoxine hydrochloride. Tablets are imprinted withM overDP in black ink on one side of the tablet and blank on the other side.
Inactive ingredients are as follows: hypromellose, magnesium stearate, magnesium trisilicate, methacrylic acid copolymer dispersion, microcrystalline cellulose, polyethylene glycol, polysorbate 80, propylene glycol and talc. In addition, the black imprinting ink contains ammonium hydroxide, black iron oxide, propylene glycol and shellac glaze.
**Doxylamine Succinate:**Doxylamine succinate is classified as an antihistamine. The chemical name for doxylamine succinate is 2-[α[2-(Dimethylamino)ethoxy]-α-methylbenzyl]pyridine succinate (1:1). The molecular formula is C17H22N2O • C4H6O4 and the molecular mass is 388.46. The structural formula is:
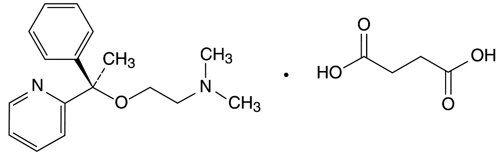
Doxylamine succinate, USP is a white or almost white powder, having a characteristic odor, that is very soluble in water and alcohol, freely soluble in chloroform and very slightly soluble in ether and benzene.
**Pyridoxine Hydrochloride:**Pyridoxine hydrochloride is a vitamin B6 analog. The chemical name for pyridoxine hydrochloride is 3,4-Pyridinedimethanol, 5-hydroxy-6-methyl-, hydrochloride. The molecular formula is C8H12ClNO3 and the molecular mass is 205.6. The structural formula is:
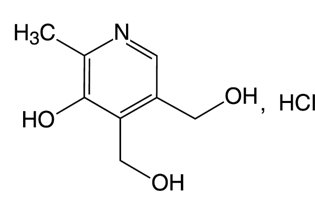
Pyridoxine hydrochloride, USP is white or practically white crystals or crystalline powder that is freely soluble in water, slightly soluble in alcohol and insoluble in ether.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Two-year carcinogenicity studies in rats and mice have been conducted with doxylamine succinate. Doxylamine succinate is not likely to have human carcinogenic potential. The carcinogenic potential of pyridoxine hydrochloride has not been evaluated.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
A double-blind, randomized, multi-center, placebo-controlled study was conducted to support the safety and efficacy of doxylamine succinate and pyridoxine hydrochloride delayed-release tablets in the treatment of nausea and vomiting of pregnancy. Adult women 18 years of age or older and 7 to 14 weeks gestation (median 9 weeks of gestation) with nausea and vomiting of pregnancy were randomized to 14 days of doxylamine succinate and pyridoxine hydrochloride delayed-release tablets or placebo. Two tablets of doxylamine succinate and pyridoxine hydrochloride were administered at bedtime on Day 1. If symptoms of nausea and vomiting persisted into the afternoon hours of Day 2, the woman was directed to take her usual dose of two tablets at bedtime that night and, beginning on Day 3, to take one tablet in the morning and two tablets at bedtime. Based upon assessment of remaining symptoms at her clinic visit on Day 4 (± 1 day), the woman may have been directed to take an additional tablet mid-afternoon. A maximum of four tablets (one in the morning, one in the mid-afternoon and two at bedtime) were taken daily.
Over the treatment period, 19% of doxylamine succinate and pyridoxine hydrochloride delayed-release tablets-treated patients remained on 2 tablets daily, 21% received 3 tablets daily, and 60% received 4 tablets daily.
The primary efficacy endpoint was the change from baseline at Day 15 in the Pregnancy Unique-Quantification of Emesis (PUQE) score. The PUQE score incorporates the number of daily vomiting episodes, number of daily heaves, and length of daily nausea in hours, for an overall score of symptoms rated from 3 (no symptoms) to 15 (most severe).
At baseline, the mean PUQE score was 9.0 in the doxylamine succinate and pyridoxine hydrochloride delayed-release tablets arm and 8.8 in the placebo arm. There was a 0.7 (95% confidence interval 0.2 to 1.2 with p-value 0.006) mean decrease (improvement in nausea and vomiting symptoms) from baseline in PUQE score at Day 15 with doxylamine succinate and pyridoxine hydrochloride delayed-release tablets compared to placebo (see Table 6).
Table 6: Change from Baseline in the Primary Endpoint, Pregnancy Unique-Quantification of Emesis (PUQE) Score at Day 15. (Intent-to-Treat Population with Last-Observation Carried Forward)
| |||
|
PUQE Score* |
Doxylamine Succinate |
Placebo |
Treatment Difference [95% Confidence Interval] |
|
Baseline Change from baseline at Day 15 |
9.0 ± 2.1 -4.8 ± 2.7 |
8.8 ± 2.1 -3.9 ± 2.6 |
-0.7 [-1.2, -0.2] |
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Doxylamine Succinate and Pyridoxine Hydrochloride Delayed-Release Tablets are available containing 10 mg of doxylamine succinate, USP and 10 mg of pyridoxine hydrochloride, USP.
The 10 mg/10 mg tablets are white to off-white, film-coated, round, unscored tablets imprinted withM overDP in black ink on one side of the tablet and blank on the other side. They are available as follows:
NDC 0378-4615-01
bottles of 100 tablets
16.2 Storage and Handling
**Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] **Keep bottle tightly closed.Protect from moisture and light. Do not remove desiccant from bottle.
Dispense in original container or equivalent air tight, light resistant container.
