Deflazacort
These highlights do not include all the information needed to use DEFLAZACORT TABLETS safely and effectively. See full prescribing information for DEFLAZACORT TABLETS. DEFLAZACORT tablets, for oral use Initial U.S. Approval: 2017
77d7525d-89c9-4fde-a6a8-0a463fd6cfeb
HUMAN PRESCRIPTION DRUG LABEL
Feb 12, 2024
Aurobindo Pharma Limited
DUNS: 650082092
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Deflazacort
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Deflazacort
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Deflazacort
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Deflazacort
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 36 mg (30 Tablets Bottle)
NDC 59651-602-30
Deflazacort Tablets
** 36 mg**
**for oral use
Rx only 30 Tablets
** AUROBINDO
****
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Deflazacort tablets are indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 5 years of age and older.
Additional pediatric use information is approved for PTC Therapeutics, Inc.'s EmflazaTM (deflazacort) tablets. However, due to PTC Therapeutics, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Deflazacort tablets are contraindicated in patients with known hypersensitivity to deflazacort or to any of the inactive ingredients. Instances of hypersensitivity, including anaphylaxis, have occurred in patients receiving corticosteroid therapy [see Warnings and Precautions (5.15) and Adverse Reactions (6.2)].
Hypersensitivity to deflazacort or any of the inactive ingredients in deflazacort tablets (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in other sections:
- Alterations in Endocrine Function [see Warnings and Precautions (5.1)]
- Immunosuppression and Increased Risk of Infection [see Warnings and Precautions (5.2)]
- Alterations in Cardiovascular/Renal Function [see Warnings and Precautions (5.3)]
- Gastrointestinal Perforation [see Warnings and Precautions (5.4)]
- Behavioral and Mood Disturbances [see Warnings and Precautions (5.5)]
- Effects on Bones [see Warnings and Precautions (5.6)]
- Ophthalmic Effects [see Warnings and Precautions (5.7)]
- Immunizations [see Warnings and Precautions (5.8)]
- Serious Skin Rashes [see Warnings and Precautions (5.9)]
- Effects on Growth and Development [see Warnings and Precautions (5.10)]
- Myopathy [see Warnings and Precautions (5.11)]
- Kaposi’s Sarcoma [see Warnings and Precautions (5.12)]
- Thromboembolic Events [see Warnings and Precautions (5.14)]
- Anaphylaxis [see Warnings and Precautions (5.15)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Study 1 [see Clinical Studies (14)], the adverse reactions that were associated with deflazacort treatment discontinuation, in decreasing order of frequency, were weight increased, obesity, cataract, and sleep disorder.
Most Common Adverse Reactions in Clinical Studies
Table 1 lists the adverse reactions that occurred in ≥5% of patients in the 0.9 mg/kg/day deflazacort-treated group and that occurred more frequently than in placebo patients in Study 1, which included patients with DMD between the ages of 5 and 15 years.
Table 1: Adverse Reactions that Occurred in ≥ 5% of Deflazacort-Treated Patients and Occurred More Frequently than in Placebo Patients with DMD (Study 1)
|
Adverse Reaction |
Deflazacort |
Placebo (N=50) |
|
Cushingoid appearance |
33 |
12 |
|
Weight increased |
20 |
6 |
|
Increased appetite |
14 |
2 |
|
Upper respiratory tract infection |
12 |
10 |
|
Cough |
12 |
6 |
|
Pollakiuria |
12 |
2 |
|
Nasopharyngitis |
10 |
6 |
|
Hirsutism |
10 |
2 |
|
Central obesity |
10 |
4 |
|
Erythema |
8 |
6 |
|
Irritability |
8 |
4 |
|
Rhinorrhea |
8 |
0 |
|
Abdominal discomfort |
6 |
2 |
1 At 12 weeks placebo patients were re-randomized to receive either deflazacort or an active comparator.
Common adverse reactions (≥ 5% of deflazacort-treated patients) that occurred over 52 weeks of exposure to deflazacort 0.9 mg/kg/day in Study 1 and at a higher rate than deflazacort 0.9 mg/kg/day in the 12-week placebo-controlled phase of the trial include Cushingoid appearance (60%), hirsutism (35%), weight increased (28%), erythema (28%), central obesity (25%), abdominal pain/abdominal pain upper (18% combined), pollakiuria (15%), constipation (10%), irritability (10%), abnormal behavior (9%), pyrexia (9%), back pain (7%), rash (7%), contusion (6%), nausea (6%), psychomotor hyperactivity (6%), epistaxis (6%), and skin striae (6%).
Study 1 also evaluated a higher dosage of deflazacort (1.2 mg/kg/day). Compared with the 0.9 mg/kg/day dosage, deflazacort 1.2 mg/kg/day over 52 weeks was associated with a higher incidence of certain adverse reactions, including Cushingoid appearance (69%), erythema (49%), hirsutism (37%), headache (34%), weight increased (32%), constipation (15%), abdominal pain upper (14%), skin striae (11%), acne (11%), and abdominal discomfort (8%). As there was no additional benefit with the 1.2 mg/kg/day dose of deflazacort, use of deflazacort 1.2 mg/kg/day is not recommended for the treatment of DMD [see Dosage and Administration (2.2)].
In an additional clinical study of two years duration with extended follow-up (Study 2), many of the same adverse reactions were observed. In addition, musculoskeletal events associated with long-term steroid use were also observed, including muscle weakness, tendon disorder, and osteopenia.
Less Common Adverse Reactions Observed in Clinical Studies
Other adverse reactions (≥ 1% frequency in any deflazacort treatment group and greater than placebo) that were observed during the 12-week placebo-controlled phase of Study 1 are shown below.
Eye Disorders: Lacrimation increased
Gastrointestinal Disorders: Dyspepsia, nausea, gastrointestinal disorder
General Disorders and Administration Site Conditions: Thirst
Infections: Hordeolum, impetigo, influenza, otitis externa, pharyngitis, tooth abscess, urinary tract infection, viral infection Injury, Poisoning and Procedural Complications: Back injury, contusion, face injury, fibula fracture, greenstick fracture, heat exhaustion
Investigations: Glucose urine present, heart rate irregular
Musculoskeletal and Connective Tissue Disorders: Back pain, muscle spasms, myalgia, neck mass, neck pain, pain in extremity
Nervous System Disorders: Dizziness, psychomotor hyperactivity
Psychiatric Disorders: Affect lability, aggression, depression, emotional disorder, middle insomnia, mood altered, mood swings, sleep disorder
Renal and Urinary Disorders: Chromaturia, dysuria, hypertonic bladder
Reproductive System and Breast Disorders: Testicular pain
Respiratory, Thoracic, and Mediastinal Disorders: Hypoventilation, rhinorrhea
Skin and Subcutaneous Tissue Disorders: Acne, alopecia, dermatitis acneiform
Vascular Disorders: Hot flush
6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of deflazacort worldwide or during post-approval use of other corticosteroids. These reactions are reported voluntarily from a population of uncertain size; therefore, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Leukocytosis
Cardiac Disorder: Heart failure
Eye Disorders: Chorioretinopathy, corneal or scleral thinning
Gastrointestinal Disorders: Acute pancreatitis (especially in children), hemorrhage, peptic ulceration, perforation of peptic ulcer
General Disorders and Administration Site Conditions: Edema, impaired healing
Immune System Disorders: Hypersensitivity including anaphylaxis
Metabolism and Nutrition Disorders: Impaired carbohydrate tolerance with increased requirement for anti-diabetic therapy, negative protein and calcium balance, potassium loss and hypokalemic alkalosis when co-administered with beta 2-agonist and xanthines
Musculoskeletal and Connective Tissue Disorders: Avascular necrosis, muscle wasting, negative nitrogen balance, tendonitis and tendon rupture when co- administered with quinolones, vertebral and long bone fractures
Nervous System Disorders: Aggravation of epilepsy, increased intra-cranial pressure with papilledema in children (pseudotumor cerebri) usually after treatment withdrawal, vertigo
Psychiatric Disorders: Anxiety, cognitive dysfunction including confusion and amnesia, delusions, hallucinations, mania, suicidal thoughts
Skin and Subcutaneous Tissue Disorders: Toxic epidermal necrolysis
Vascular Disorders: Thromboembolism, in particular in patients with underlying conditions associated with increased thrombotic tendency, benign intracranial hypertension
The most common adverse reactions (≥ 10% for deflazacort****and greater than placebo) are Cushingoid appearance, weight increased, increased appetite, upper respiratory tract infection, cough, pollakiuria, hirsutism, central obesity, and nasopharyngitis (6.1)
** To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or****www.fda.gov/medwatch.**
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors and Inducers
Moderate or Strong CYP3A4 Inhibitors
The active metabolite of deflazacort, 21-desDFZ, is a substrate of CYP3A4 [see Clinical Pharmacology (12.3)]. Co-administration of deflazacort with clarithromycin, a strong CYP3A4 inhibitor, increased total exposure to 21-desDFZ by about 3-fold. Therefore, give one third the recommended dosage of deflazacort when moderate or strong CYP3A4 inhibitors (e.g., clarithromycin, fluconazole, diltiazem, verapamil, grapefruit juice) are used concomitantly with deflazacort [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
Moderate or Strong CYP3A4 Inducers
Co-administration of deflazacort with rifampin, a strong CYP3A4 inducer, significantly decreased the exposure of 21-desDFZ. Avoid concomitant use of strong (e.g., efavirenz) or moderate (e.g., carbamazepine, phenytoin) CYP3A4 inducers with deflazacort [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
7.2 Neuromuscular Blockers
Patients receiving corticosteroids, including deflazacort, and concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium) may be at increased risk of developing an acute myopathy [see Warnings and Precautions (5.11)].
- Moderate or strong CYP3A4 inhibitors: Give one third of the recommended dosage of deflazacort****(7.1)
- Avoid use of moderate or strong CYP3A4 inducers with deflazacort, as they may reduce efficacy (7.1)
Additional pediatric use information is approved for PTC Therapeutics, Inc.'s EmflazaTM (deflazacort) tablets. However, due to PTC Therapeutics, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
- 6 mg: White to off-white, uncoated round shaped biconvex tablets, debossed with “DL6” on one side and plain on other side.
- 18 mg: White to off-white, uncoated round shaped biconvex tablets, debossed with “DL18” on one side and plain on other side.
- 30 mg: White to off-white, uncoated oval shaped biconvex tablets, debossed with “DL30” on one side and plain on other side.
- 36 mg: White to off-white, uncoated oval shaped biconvex tablets, debossed with “DL36” on one side and plain on other side.
OVERDOSAGE SECTION
10 OVERDOSAGE
Treatment of acute overdosage is by immediate gastric lavage or emesis followed by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy, the dosage of deflazacort may be reduced temporarily, or alternate day treatment may be introduced.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Deflazacort is a corticosteroid prodrug, whose active metabolite, 21-desDFZ, acts through the glucocorticoid receptor to exert anti-inflammatory and immunosuppressive effects. The precise mechanism by which deflazacort exerts its therapeutic effects in patients with DMD is unknown.
12.3 Pharmacokinetics
Absorption
After oral administration in the fasted state, the median Tmax with deflazacort tablets is about 1 hour (range 0.25 to 2 hours).
Food Effect: Co-administration of deflazacort tablets with a high-fat meal reduced Cmax by about 30% and delayed Tmax by one hour, relative to administration under fasting conditions, but there was no effect on the overall systemic absorption as measured by AUC. The bioavailability of deflazacort tablets was similar to that of the oral suspension. The administration of deflazacort with food or crushed in applesauce did not affect the absorption and bioavailability of deflazacort.
Distribution
The protein binding of the active metabolite of deflazacort is about 40%.
Elimination
Metabolism
Deflazacort is rapidly converted to the active metabolite 21-desDFZ by esterases after oral administration. 21-desDFZ is further metabolized by CYP3A4 to several other inactive metabolites, including 6β-hydroxy-21-desacetyl deflazacort.
Excretion
Urinary excretion is the predominant route of deflazacort elimination (about 68% of the dose), and the elimination is almost completed by 24 hours post dose. 21-desDFZ accounts for 18% of the eliminated drug in the urine.
Specific Populations
Pediatric Patients
The Cmax values (Geometric mean, %CV) of 21-desDFZ in children (ages 4 to 11, N=16) and adolescents (ages 12 to 16, N=8) was 206 ng/mL (95.6%) and 381 ng/mL (37.7%), respectively, on Day 1 after administration of 0.9 mg/kg deflazacort. The AUCinf (Geometric mean, %CV) of 21-desDFZ in children (ages 4 to 11, N=16) and adolescents (ages 12 to 16, N=8) was 400 ng•h/mL (87.5%) and 655 ng•h/mL (58.1%) on Day 1 after administration of 0.9 mg/kg deflazacort.
Male and Female Patients
There are no differences in the pharmacokinetics of 21-desDFZ between males and females.
Racial or Ethnic Groups
There are no differences in the pharmacokinetics of 21-desDFZ between Caucasians and non-Caucasians.
Patients with Renal Impairment
In a study (N=16) comparing subjects with end stage renal disease (creatinine clearance less than 15 mL/min) with healthy matched controls, 21-desDFZ exposure was similar between the groups.
Patients with Hepatic Impairment
In a study (N=16) comparing subjects with moderate hepatic impairment (Child- Pugh Class B) with healthy matched controls, 21-desDFZ exposure was similar between the groups. There is no experience in patients with severe hepatic impairment.
Drug Interaction Studies
In Vivo Assessment of Drug Interactions
Compared to administration of deflazacort alone, administration of deflazacort following multiple doses of a strong CYP3A4 and Pgp inhibitor (clarithromycin) resulted in markedly higher Cmax, AUClast, and AUCinf values of 21-desDFZ. Geometric mean exposure (Cmax, AUClast, and AUCinf) of 21-desDFZ ranged from 2.3-fold to 3.4-fold higher following administration of clarithromycin [see Dosage and Administration (2.5)].
Compared to administration of deflazacort alone, administration of deflazacort following multiple doses of a strong CYP3A4 inducer (rifampicin) resulted in markedly lower Cmax, AUClast, and AUCinf values of 21-desDFZ. Geometric mean exposures (Cmax, AUClast, and AUCinf) of 21-desDFZ were approximately 95% lower following administration of rifampin [see Drug Interactions (7.1)].
6β-Hydroxy-21-desacetyl deflazacort, a secondary and inactive metabolite, is not expected to cause any clinically meaningful interactions with the CYP enzymes or transporters.
In Vitro Assessment of Drug Interactions
Drug-Metabolizing Enzyme Inhibition
21-desDFZ at clinically relevant concentrations did not inhibit CYP1A2, 2C9, 2C19, 3A4, UGT1A1, UGT1A4, UGT1A6, UGT1A9, or UGT2B7 and exhibited weak and not likely clinically meaningful inhibition for 2B6, 2C8, 2D6, and 3A4, UGT1A3 and UGT2B15.
6β-Hydroxy-21-desacetyl deflazacort at clinically relevant concentrations did not significantly inhibit CYP2C19, 3A4 1A2, 2B6, 2C8, 2C9, or 2D6.
Drug-Metabolizing Enzyme Induction
21-desDFZ and 6β-hydroxy-21-desacetyl deflazacort at clinically relevant concentrations did not significantly induce CYP1A2, 2B6, or 3A4.
Transporters
Both deflazacort and 21-desDFZ are substrates of Pgp. 21-desDFZ is not a substrate for BCRP. Neither deflazacort nor 21-desDFZ inhibited Pgp or BCRP in vitro. 21-desDFZ was not a substrate for SLC transporters OATP1B1 or OATP1B3, and did not inhibit SLC transporters OATP1B1, OATP1B3, OAT1, OAT3, or OCT2.
6β-Hydroxy-21-desacetyl deflazacort at clinically relevant concentrations did not significantly inhibit BCRP, OAT1, OAT3, Pgp, OATP1B1, OATP1B3 MATE1, MATE2-K, OCT1, OCT2, or BSEP transporters.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Assessments Prior to First Dose of Deflazacort Tablets
Administer all immunizations according to immunization guidelines prior to starting deflazacort tablets. Administer live-attenuated or live vaccines at least 4 to 6 weeks prior to starting deflazacort tablets [see Warnings and Precautions (5.8)].
2.2 Dosing Information
The recommended oral dosage of deflazacort tablets are approximately 0.9 mg/kg/day once daily. If tablets are used, round up to the nearest possible dose. Any combination of the four deflazacort tablet strengths can be used to achieve this dose.
2.3 Discontinuation
Dosage of deflazacort tablets must be decreased gradually if the drug has been administered for more than a few days [see Warnings and Precautions (5.1)].
2.4 Important Preparation and Administration Instructions
Deflazacort tablets can be taken with or without food. Do not administer deflazacort tablets with grapefruit juice [see Drug Interactions (7.1)].
Deflazacort tablets can be administered whole or crushed and taken immediately after mixing with applesauce.
2.5 Dosage Modification for Use with CYP3A4 Inhibitors and Inducers
CYP3A4 Inhibitors
Give one third of the recommended dosage when deflazacort tablets are administered with moderate or strong CYP3A4 inhibitors. For example, a 36 mg per day dose would be reduced to a 12 mg per day dose when used with moderate or strong CYP3A4 inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
CYP3A4 Inducers
Avoid use with moderate or strong CYP3A4 inducers with deflazacort tablets [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born to mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism. There are no adequate and well-controlled studies with deflazacort in pregnant women to inform drug- associated risks.
Corticosteroids, including deflazacort, readily cross the placenta. Adverse developmental outcomes, including orofacial clefts (cleft lip, with or without cleft palate) and intrauterine growth restriction, and decreased birth weight, have been reported with maternal use of corticosteroids, including deflazacort, during pregnancy. Some epidemiologic studies report an increased risk of orofacial clefts from about 1 per 1000 infants to 3 to 5 per 1000 infants; however, a risk for orofacial clefts has not been observed in all studies. Intrauterine growth restriction and decreased birth weight appear to be dose-related; however, the underlying maternal condition may also contribute to these risks (see Data). The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal reproduction studies have not been conducted with deflazacort. Animal reproduction studies conducted with other corticosteroids in pregnant mice, rats, hamsters, and rabbits using clinically relevant doses have shown an increased incidence of cleft palate. An increase in embryofetal death, intrauterine growth retardation, and constriction of the ductus arteriosus were observed in some animal species.
Data
Human Data
Multiple cohort and case-controlled studies in humans suggest that maternal corticosteroid use during the first trimester increases the rate of cleft lip, with or without cleft palate, from about 1/1000 infants to 3 to 5/1000 infants. Two prospective case-controlled studies showed decreased birth weight in infants exposed to maternal corticosteroids in utero.
8.2 Lactation
Risk Summary
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for deflazacort and any potential adverse effects on the breastfed infant from deflazacort. There are no data on the effects on milk production.
8.4 Pediatric Use
The safety and effectiveness of deflazacort for the treatment of DMD have been established in patients 5 years of age and older. Use of deflazacort in pediatric patients is supported by a multicenter, randomized, double-blind, placebo- and active-controlled study in 196 males 5 to 15 years of age [see Clinical Studies (14)].
Safety and effectiveness in pediatric patients below the age of 2 years have not been established.
Juvenile Animal Toxicity Data
Oral administration of deflazacort (0, 0.1, 0.3, and 1.0 mg/kg/day) to juvenile rats from postnatal day (PND) 21 to 80 resulted in decreased body weight gain and adverse effects on skeletal development (including decreased cellularity of growth plate and altered bone distribution) and on lymphoid tissue (decreased cellularity). A no-effect dose was not identified. In addition, neurological and neurobehavioral abnormalities were observed at the mid and/or high dose. Plasma 21-desDFZ exposure (AUC) at the lowest dose tested (0.1 mg/kg/day) was lower than that in humans at the recommended human dose of deflazacort (0.9 mg/kg/day).
Additional pediatric use information is approved for PTC Therapeutics, Inc.'s EmflazaTM (deflazacort) tablets. However, due to PTC Therapeutics, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
DMD is largely a disease of children and young adults; therefore, there is no geriatric experience with deflazacort.
8.6 Renal Impairment
No dose adjustment is required in patients with mild, moderate or severe renal impairment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dose adjustment is required in patients with mild or moderate hepatic impairment [see Clinical Pharmacology (12.3)]. There is no clinical experience in patients with severe hepatic impairment, and a dosing recommendation cannot be provided for patients with severe hepatic impairment.
DESCRIPTION SECTION
11 DESCRIPTION
The active ingredient in deflazacort tablets is deflazacort (a corticosteroid). Corticosteroids are adrenocortical steroids, both naturally occurring and synthetic. The molecular formula for deflazacort is C25H31NO6. The chemical name for deflazacort is (11β,16β)-21-(acetyloxy)-11-hydroxy-2'-methyl-5'H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione, and the structure is:
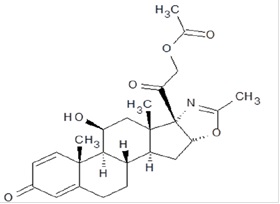
Deflazacort is a white to off-white, odourless fine powder and has a molecular weight of 441.517. Deflazacort is freely soluble in acetic acid and dichloromethane; soluble in methanol and acetone.
Deflazacort tablets for oral administration is available as an immediate- release tablet in strengths of 6, 18, 30 and 36 mg. Each tablet contains deflazacort and the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, polysorbate 80, and pregelatinized starch (maize).
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a published 2-year carcinogenicity study in rats, oral administration of deflazacort (0, 0.03, 0.06, 0.12, 0.25, 0.50, or 1 mg/kg/day) resulted in bone tumors (osteosarcoma and osteoma) of the head at 0.25 mg/kg/day, the highest evaluable dose. Doses higher than 0.25 mg/kg/day could not be evaluated for tumors because of a marked decrease in survival.
In a 6-month carcinogenicity study in transgenic (Tg.RasH2) mice, oral administration of deflazacort (0, 2, 5, or 20 mg/kg/day in males; 0, 0.5, 2, or 5 mg/kg/day in females) resulted in an increase in stomach tumors (adenoma) at the highest dose tested in males and females.
Mutagenesis
Deflazacort and 21-desDFZ were negative in in vitro (bacterial reverse mutation and human lymphocyte chromosomal aberration) assays and deflazacort was negative in an in vivo (rat micronucleus) assay.
Impairment of Fertility
Fertility studies in animals were not conducted with deflazacort. No effects on the male reproductive system were observed following oral administration of deflazacort to monkeys (0, 1, 3, or 6 mg/kg/day) for 39 weeks or rats (0, 0.05, 0.15, or 0.5 mg/kg/day) for 26 weeks. Plasma 21-desDFZ exposures (AUC) at the highest doses tested in monkey and rat were 4 and 2 times, respectively, that in humans at the recommended human dose of deflazacort (0.9 mg/kg/day).
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The effectiveness of deflazacort for the treatment of DMD was established in Study 1, a multicenter, randomized, double-blind, placebo-controlled, 52-week study conducted in the US and Canada. The study population consisted of 196 male pediatric patients 5 to 15 years of age with documented mutation of the dystrophin gene, onset of weakness before 5 years of age, and serum creatinine kinase activity at least 10 times the upper limit of normal (ULN) at some stage in their illness. Patients were randomized to therapy with deflazacort (0.9 or 1.2 mg/kg/day), an active comparator, or placebo. A comparison to placebo was made after 12 weeks of treatment. After 12 weeks, placebo patients were re-randomized to receive either deflazacort or the active comparator; all patients continued treatment for an additional 40 weeks. Baseline characteristics were comparable between the treatment arms.
In Study 1, efficacy was evaluated by assessing the change between Baseline and Week 12 in average strength of 18 muscle groups. Individual muscle strength was graded using a modified Medical Research Council (MRC) 11-point scale, with higher scores representing greater strength.
The change in average muscle strength score between Baseline and Week 12 was significantly greater for the deflazacort 0.9 mg/kg/day dose group than for the placebo group (see Table 2).
Table 2: Analysis of Change from Baseline at Week 12 in Average Muscle Strength Score (Study 1)
|
Treatment |
N |
Change from Baseline |
P-value |
|
Deflazacort 0.9 mg/kg/day |
51 |
0.15 (0.01, 0.28) |
0.017 |
|
Placebo |
50 |
-0.10 (-0.23, 0.03) |
Compared with the deflazacort 0.9 mg/kg/day group, the deflazacort 1.2 mg/kg/day group demonstrated a small additional benefit compared to placebo at Week 12, but had a greater incidence of adverse reactions. Therefore, use of a 1.2 mg/kg/day dosage of deflazacort is not recommended [see Dosage and Administration (2.2)].
Although not a pre-specified statistical analysis, compared with placebo, the deflazacort 0.9 mg/kg/day dose group demonstrated at Week 52 the persistence of the treatment effect observed at Week 12 and the small advantage of the 1.2 mg/kg/day dose that was observed at Week 12 was no longer present. Also not statistically controlled for multiple comparisons, results on several timed measures of patient function (i.e., time to stand from supine, time to climb 4 stairs, and time to walk or run 30 feet) numerically favored deflazacort 0.9 mg/kg/day at Week 12, in comparison with placebo.
An additional randomized, double-blind, placebo-controlled, 104-week clinical trial evaluated deflazacort in comparison to placebo (Study 2). The study population consisted of 29 male children 6 to 12 years of age with a DMD diagnosis confirmed by the documented presence of abnormal dystrophin or a confirmed mutation of the dystrophin gene. The results of the analysis of the primary endpoint of average muscle strength scores in Study 2 (graded on a 0 to 5 scale) at 2 years were not statistically significant, possibly because of a limited number of patients remaining in the placebo arm (subjects were discontinued from the trial when they lost ambulation). Although not statistically controlled for multiple comparisons, average muscle strength scores at Months 6 and 12, as well as the average time to loss of ambulation, numerically favored deflazacort in comparison with placebo.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Deflazacort Tablets, 6 mg are white to off-white, uncoated round shaped biconvex tablets, debossed with “DL6” on one side and plain on other side. They are supplied as follows:
Bottles of 100 NDC 59651-599-01
Deflazacort Tablets, 18 mg are white to off-white, uncoated round shaped biconvex tablets, debossed with “DL18” on one side and plain on other side. They are supplied as follows:
Bottles of 30 NDC 59651-600-30
Deflazacort Tablets, 30 mg are white to off-white, uncoated oval shaped biconvex tablets, debossed with “DL30” on one side and plain on other side. They are supplied as follows:
Bottles of 30 NDC 59651-601-30
Deflazacort Tablets, 36 mg are white to off-white, uncoated oval shaped biconvex tablets, debossed with “DL36” on one side and plain on other side. They are supplied as follows:
Bottles of 30 NDC 59651-602-30
16.2 Storage and Handling
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Administration
- Warn patients and/or caregivers to not stop taking deflazacort tablets abruptly or without first checking with their healthcare providers as there may be a need for gradual dose reduction to decrease the risk of adrenal insufficiency [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
- Deflazacort tablets may be taken with or without food. Do not take deflazacort tablets with grapefruit juice.
- Deflazacort tablets may be taken whole or crushed and taken immediately after mixing with applesauce.
Increased Risk of Infection
Tell patients and/or caregivers to inform their healthcare provider if the patient has had recent or ongoing infections or if they have recently received a vaccine. Medical advice should be sought immediately if the patient develops fever or other signs of infection. Patients and/or caregivers should be made aware that some infections can potentially be severe and fatal.
Warn patients who are on corticosteroids to avoid exposure to chickenpox or measles and to alert their healthcare provider immediately if they are exposed [see Warnings and Precautions (5.2)].
Alterations in Cardiovascular/Renal Function
Inform patients and/or caregivers that deflazacort tablets can cause an increase in blood pressure and water retention. If this occurs, dietary salt restriction and potassium supplementation may be needed [see Warnings and Precautions (5.3)].
Behavioral and Mood Disturbances
Advise patients and/or caregivers about the potential for severe behavioral and mood changes with deflazacort tablets and encourage them to seek medical attention if psychiatric symptoms develop [see Warnings and Precautions (5.5)].
Decreases in Bone Mineral Density
Advise patients and/or caregivers about the risk of osteoporosis with prolonged use of deflazacort tablets, which can predispose the patient to vertebral and long bone fractures [see Warnings and Precautions (5.6)].
Ophthalmic Effects
Inform patients and/or caregivers that deflazacort tablets may cause cataracts or glaucoma and advise monitoring if corticosteroid therapy is continued for more than 6 weeks [see Warnings and Precautions (5.7)].
Vaccination
Advise patients and/or caregivers to bring immunizations up-to-date according to immunization guidelines prior to starting therapy with deflazacort tablets. Live-attenuated or live vaccines should be administered at least 4 to 6 weeks prior to starting deflazacort tablets. Inform patients and/or caregivers that they may receive concurrent vaccinations with use of deflazacort tablets, except for live-attenuated or live vaccines. [see Warnings and Precautions (5.8)].
Serious Skin Rashes
Instruct patients and/or caregivers to seek medical attention at the first sign of a rash [see Warnings and Precautions (5.9)].
Drug Interactions
Certain medications can cause an interaction with deflazacort tablets. Advise patients and/or caregivers to inform their healthcare provider of all the medicines the patient is taking, including over-the-counter medicines (such as insulin, aspirin or other NSAIDS), dietary supplements, and herbal products. Inform patients and/or caregivers that alternate therapy, dosage adjustment, and/or special test(s) may be needed during the treatment.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Issued: February 2023
